Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus
- PMID: 18678911
- PMCID: PMC2495015
- DOI: 10.1073/pnas.0804985105
Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus
Abstract
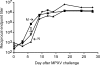
The success of the World Health Organization smallpox eradication program three decades ago resulted in termination of routine vaccination and consequent decline in population immunity. Despite concerns regarding the reintroduction of smallpox, there is little enthusiasm for large-scale redeployment of licensed live vaccinia virus vaccines because of medical contraindications and anticipated serious side effects. Therefore, highly attenuated strains such as modified vaccinia virus Ankara (MVA) are under evaluation in humans and animal models. Previous studies showed that priming and boosting with MVA provided protection for >2 years in a monkeypox virus challenge model. If variola virus were used as a biological weapon, however, the ability of a vaccine to quickly induce immunity would be essential. Here, we demonstrate more rapid immune responses after a single vaccination with MVA compared to the licensed Dryvax vaccine. To determine the kinetics of protection of the two vaccines, macaques were challenged intravenously with monkeypox virus at 4, 6, 10, and 30 days after immunization. At 6 or more days after vaccination with MVA or Dryvax, the monkeys were clinically protected (except for 1 of 16 animals vaccinated with MVA), although viral loads and number of skin lesions were generally higher in the MVA vaccinated group. With only 4 days between immunization and intravenous challenge, however, MVA still protected whereas Dryvax failed. Protection correlated with the more rapid immune response to MVA compared to Dryvax, which may be related to the higher dose of MVA that can be tolerated safely.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
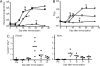
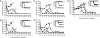
References
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and Its Eradication. Geneva: World Health Organization; 1988.
-
- Henderson DA, et al. Smallpox as a biological weapon: Medical and public health management. J Am Med Assoc. 1999;281:2127–2137. - PubMed
-
- Lane JM, Goldstein J. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann Int Med. 2003;138:488–493. - PubMed
-
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: A review, part II. Adverse events. Clin Inf Dis. 2003;37:251–271. - PubMed
-
- Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

