High crystallizability under air-exclusion conditions of the full-length LysR-type transcriptional regulator TsaR from Comamonas testosteroni T-2 and data-set analysis for a MIRAS structure-solution approach
- PMID: 18678953
- PMCID: PMC2494969
- DOI: 10.1107/S1744309108019738
High crystallizability under air-exclusion conditions of the full-length LysR-type transcriptional regulator TsaR from Comamonas testosteroni T-2 and data-set analysis for a MIRAS structure-solution approach
Abstract
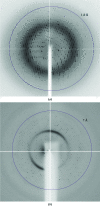
The full-length LysR-type transcriptional regulator TsaR from Comamonas testosteroni T-2 was heterologously overexpressed in Escherichia coli, purified and stabilized under conditions that favoured its rapid crystallization using the microbatch-under-oil technique. The purified protein was highly crystallizable and two different crystal forms were readily obtained. However, only monoclinic crystals gave diffraction beyond 2 A and there was a slight variation in unit-cell parameters between crystals. The only other LysR-type regulator for which a full-length crystal form is available is CbnR, but no solution could be obtained when this was used as a model in molecular replacement. Mercury and xenon derivatives were therefore produced in order to phase the structure using a MIRAS approach.
Figures


References
-
- Clark, T., Haddad, S., Neidle, E. & Momany, C. (2004). Acta Cryst. D60, 105–108. - PubMed
-
- Cook, A. M., Laue, H. & Junker, F. (1999). FEMS Microbiol. Rev.22, 399–419. - PubMed
-
- Ezezika, O. C., Haddad, S., Clark, T. J., Neidle, E. L. & Momany, C. (2007). J. Mol. Biol.367, 616–629. - PubMed
-
- García-Ruiz, J. M. (2003). Methods Enzymol.368, 130–154. - PubMed
-
- Garman, E. F. & Schneider, T. R. (1997). J. Appl. Cryst.30, 211–237.

