Sulfonylurea receptor 1 in the germinal matrix of premature infants
- PMID: 18679166
- PMCID: PMC2647511
- DOI: 10.1203/PDR.0b013e318186e5a9
Sulfonylurea receptor 1 in the germinal matrix of premature infants
Abstract
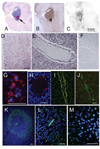
Germinal matrix (GM) hemorrhage (GMH) is a major cause of mortality and of life-long morbidity from cerebral palsy. GMH is typically preceded by hypoxic/ischemic events and is believed to arise from rupture of weakened veins in the GM. In the CNS, hypoxia/ischemia up-regulate sulfonylurea receptor 1 (SUR1)-regulated NCCa-ATP channels in microvascular endothelium, with channel activation by depletion of ATP being responsible for progressive secondary hemorrhage. We hypothesized that this channel might be up-regulated in the GM of preterm infants at risk for GMH. Here, we studied expression of the regulatory subunit of the channel, SUR1, and its transcriptional antecedent, hypoxia inducible factor 1 (HIF1), in postmortem tissues of premature infants who either were at risk for or who sustained GMH. We found regionally specific up-regulation of HIF1 and of SUR1 protein and mRNA in GM tissues, compared with remote cortical tissues. Up-regulation was prominent in most progenitor cells, whereas in veins, SUR1 was found predominantly in infants who had sustained GMH compared with those without hemorrhage. Our data suggest that the SUR1-regulated NCCa-ATP channel may be associated with GMH, and that pharmacological block of these channels could potentially reduce the incidence of this devastating complication of prematurity.
Conflict of interest statement
Conflict of interest: JMS holds a US patent (# 7,285,574), "A novel non-selective cation channel in neural cells and methods for treating brain swelling".
Figures

References
-
- Vergani P, Locatelli A, Doria V, Assi F, Paterlini G, Pezzullo JC, Ghidini A. Intraventricular hemorrhage and periventricular leukomalacia in preterm infants. Obstet Gynecol. 2004;104:225–231. - PubMed
-
- Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–949. - PubMed
-
- Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–1090. - PubMed
-
- Lou HC. On the pathogenesis of germinal layer hemorrhage in the neonate. APMIS. 1993;40 Suppl:97–102. - PubMed
-
- Levy ML, Masri LS, McComb JG. Outcome for preterm infants with germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;41:1111–1117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

