PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma
- PMID: 18679424
- PMCID: PMC2813858
- DOI: 10.1038/onc.2008.255
PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma
Abstract
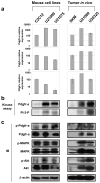
Alveolar rhabdomyosarcoma is an aggressive skeletal muscle cancer of childhood. Our initial studies of rhabdomyosarcoma gene expression for patients enrolled in a national clinical trial suggested that platelet-derived growth factor receptor A (PDGFR-A) may be a mediator of disease progression and metastasis. Using our conditional mouse tumor models that authentically recapitulate the primary mutations and metastatic progression of alveolar rhabdomyosarcomas in humans, we found by immunoblotting and immunokinase assays that PDGFR-A and its downstream effectors, mitogen-activated protein kinase and Akt, were highly activated in both primary and metastatic tumors. Inhibition of PDGFR-A by RNA interference, small molecule inhibitor or neutralizing antibody had a dramatic effect on tumor cell growth both in vitro and in vivo, although resistance evolved in one-third of tumors. These results establish proof-of-principal for PDGFR-A as a therapeutic target in alveolar rhabdomyosarcoma.
Figures





References
-
- Armistead PM, Salganick J, Roh JS, Steinert DM, Patel S, Munsell M, et al. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma: correlation with overall survival in 105 patients. Cancer. 2007;110:2293–303. - PubMed
-
- Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–52. - PubMed
-
- Blandford MC, Barr FG, Lynch JC, Randall RL, Qualman SJ, Keller C. Rhabdomyosarcomas utilize developmental, myogenic growth factors for disease advantage: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2006;46:329–38. - PubMed
-
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. - PubMed
-
- Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int. 2000;57:908–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

