EvoD/Vo: the origins of BMP signalling in the neuroectoderm
- PMID: 18679435
- PMCID: PMC2888941
- DOI: 10.1038/nrg2417
EvoD/Vo: the origins of BMP signalling in the neuroectoderm
Abstract
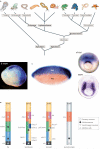
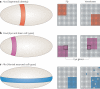
The genetic systems controlling body axis formation trace back as far as the ancestor of diploblasts (corals, hydra, and jellyfish) and triploblasts (bilaterians). Comparative molecular studies, often referred to as evo-devo, provide powerful tools for elucidating the origins of mechanisms for establishing the dorsal-ventral and anterior-posterior axes in bilaterians and reveal differences in the evolutionary pressures acting upon tissue patterning. In this Review, we focus on the origins of nervous system patterning and discuss recent comparative genetic studies; these indicate the existence of an ancient molecular mechanism underlying nervous system organization that was probably already present in the bilaterian ancestor.
Figures



References
Highlighted references
-
-
Shows that BMPs act in a dose-dependent fashion to repress the expression of neural genes in dorsal and lateral regions of the Drosophila melanogaster embryo; its also proposes that this may be a conserved mechanism for neural patterning.
-
-
- Denes AS, et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–88. - PubMed
-
-
Reveals remarkable similarities in the dorsal-ventral organization of cell markers and cell types in the CNS of annelid worms and vertebrates.
-
-
- Geoffroy St.-Hilaire E. Considérations générales sur la vertèbre (Translation: General considerations on vertebrates) Mém. Mus. Hist. Nat. 1822;9:89–119.
References
-
- Dunn CW, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9. - PubMed
-
- Valentine JW. On The Origin of Phyla. Vol. 614. University of Chicago Press; Chicago: 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

