Study of the impact of oseltamivir on the risk for pneumonia and other outcomes of influenza, 2000-2005
- PMID: 18679536
- PMCID: PMC2491667
Study of the impact of oseltamivir on the risk for pneumonia and other outcomes of influenza, 2000-2005
Abstract
Context: Influenza results in large numbers of secondary complications and hospitalizations.
Objective: To assess the impact of oseltamivir on influenza-related complications and hospitalizations by analyzing health insurance claims data for 6 influenza seasons.
Design: A retrospective cohort study utilizing claims data from the 2000-2005 influenza seasons.
Setting: Claims data were obtained from Thomson Healthcare MarketScan Research Databases.
Patients: Patients prescribed oseltamivir within 1 day of influenza diagnosis were compared to those prescribed no antiviral therapy (controls).
Outcomes: Frequencies of pneumonia, other respiratory illnesses, and otitis media, and rates of hospitalization, were compared for the oseltamivir and no antiviral groups. Expenditure was also analyzed. Relative risk (RR) for each outcome was assessed using Cox proportional hazards regression.
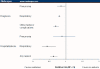
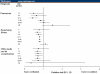
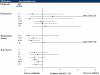
Results: Overall, 31,674 patients received oseltamivir and were propensity matched to patients with no antiviral prescription. Oseltamivir reduced the risk of diagnosis of pneumonia by 15% (RR 0.85, 95% confidence interval [CI] 0.73, 0.98), other respiratory illnesses by 20% (RR 0.80, 95% CI: 0.76, 0.83), and otitis media and its complications by 31% (RR 0.69, 95% CI: 0.61, 0.79). The greatest reductions in pneumonia risk, of 57% and 52%, were observed for children aged 6-12 years (RR 0.43, 95% CI: 0.26, 0.71) and 1-2 years (RR 0.48, 95% CI: 0.24, 0.99), respectively. Overall, hospitalization rates were reduced by 38% (RR 0.62, 95% CI: 0.52, 0.74) in the oseltamivir group compared with the no antiviral group. Total adjusted expenditures in the oseltamivir and no antiviral groups were not significantly different.
Conclusions: Oseltamivir reduced the relative risk of influenza-related complications and hospitalization when prescribed immediately upon presentation of influenza.
Figures

References
-
- Abdel-Magid AF, Maryanoff CA, Mehrman SJ. Synthesis of influenza neuraminidase inhibitors. Curr Opin Drug Discov Devel. 2001;4:776–791. - PubMed
-
- Smith NM, Bresee JS, Shay DK, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. - PubMed
-
- Zimmerman RK, Ruben FL, Ahwesh ER. Influenza, influenza vaccine, and amantadine/rimantadine. J Fam Pract. 1997;45:107–122. quiz 23–24. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
