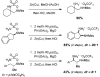
Catalytic C-H amination for the preparation of substituted 1,2-diamines
- PMID: 18680286
- PMCID: PMC2680474
- DOI: 10.1021/ja803344v
Catalytic C-H amination for the preparation of substituted 1,2-diamines
Abstract
Rhodium-catalyzed C-H insertion of hydroxylamine-derived sulfamate esters makes possible the synthesis of unique oxathiadiazinane heterocycles, which upon mild reduction furnish differentially substituted 1,2-diamine products. This highly chemo- and diastereoselective transformation underscores the power of catalytic C-H functionalization as a general approach to C-N bond construction.
Figures
References
-
-
For general applications, see: Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug Des. 2006;67:101–114.. de Parrodi CA, Juaristi E. Synlett. 2006;17:2699–2715..
-
-
- Lucet D, Le Gall T, Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580–2627. - PubMed
- Westermann B. Angew. Chem., Int. Ed. 2003;42:151–153. - PubMed
- Savoia D. Top. Organomet. Chem. 2005;15:1–58.
- Muñiz K. New J. Chem. 2005;29:1371–1385.
- Mortensen MS, O'Doherty GA. Chemtracts: Org. Chem. 2005;18:555–561.
- Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308–7309. - PubMed
- Ichikawa Y, Egawa H, Ito T, Isobe M, Nakano K, Kotsuki H. Org. Lett. 2006;8:5737–5740. - PubMed
- Fritz JA, Nakhla JS, Wolfe JP. Org. Lett. 2006;8:2531–2534. - PMC - PubMed
- Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496–7497. - PubMed
- Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943–4945. - PubMed
- Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035–2038. - PMC - PubMed
- Schomaker JM, Boyd WC, Stewart IC, Toste FD, Bergman RG. J. Am. Chem. Soc. 2008;130:3777–3779. - PubMed
- Singh A, Johnston JN. J. Am. Chem. Soc. 2008;130:5866–5867. - PubMed
- Muñiz K, Hövelmann CH, Streuff J, Campos-Gómez E. Pure Appl. Chem. 2008;80:1089–1096. and references therein.
-
- Espino CG, Wehn PM, Chow J, Du Bois J. J. Am. Chem. Soc. 2001;123:6935–6936.
-
For recent reviews on C—H amination, see: Müller P, Fruit C. Chem. Rev. 2003;103:2905–2920.. Halfen JA. Curr. Org. Chem. 2005;9:657–669.. Davies HML, Manning JR. Nature. 2008;451:417–424.
-
-
We refer to these heterocycles as oxathiadiazinanes for convenience. We are aware of only one prior report of such compounds; see: Arfaei A, Smith S. J. Chem. Soc., Perkin Trans. 1. 1984:1791–1794.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases