The hepcidin-binding site on ferroportin is evolutionarily conserved
- PMID: 18680715
- PMCID: PMC2660598
- DOI: 10.1016/j.cmet.2008.07.002
The hepcidin-binding site on ferroportin is evolutionarily conserved
Retraction in
-
Retraction notice to: The hepcidin-binding site on ferroportin is evolutionarily conserved.Cell Metab. 2014 Jun 3;19(6):1067. doi: 10.1016/j.cmet.2014.05.010. Cell Metab. 2014. PMID: 25025111 Free PMC article. No abstract available.
Abstract
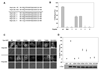
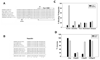
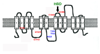
Mammalian iron homeostasis is regulated by the interaction of the liver-produced peptide hepcidin and its receptor, the iron transporter ferroportin. Hepcidin binds to ferroportin resulting in degradation of ferroportin and decreased cellular iron export. We identify the hepcidin-binding domain (HBD) on ferroportin and show that a synthetic 19 amino acid peptide corresponding to the HBD recapitulates the characteristics and specificity of hepcidin binding to cell-surface ferroportin. The binding of mammalian hepcidin to ferroportin or the HBD shows an unusual temperature dependency with an increased rate of dissociation at temperatures below 15 degrees C. The increased rate of dissociation is due to temperature- dependent changes in hepcidin structure. In contrast, hepcidin from poikilothermic vertebrates, such as fish or frogs, binds the HBD in a temperature-independent fashion. The affinity of hepcidin for the HBD permits a rapid, sensitive assay of hepcidin from all species and yields insights into the evolution of hepcidin.
Figures




Comment in
-
Regulation of Iron Homeostasis: Is It All in the HBD?Gastroenterology. 2009 Apr;136(4):1449-51. doi: 10.1053/j.gastro.2009.02.034. Epub 2009 Feb 24. Gastroenterology. 2009. PMID: 19245872 No abstract available.
References
-
- Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P. Serum ferritin and ageing. Age Ageing. 1981;10:119–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

