Palladium-catalyzed cross-coupling reactions of organosilanols and their salts: practical alternatives to boron- and tin-based methods
- PMID: 18681465
- PMCID: PMC2648401
- DOI: 10.1021/ar800037p
Palladium-catalyzed cross-coupling reactions of organosilanols and their salts: practical alternatives to boron- and tin-based methods
Abstract
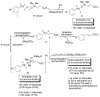
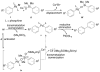
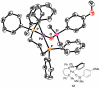
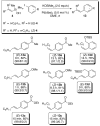
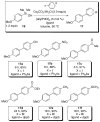
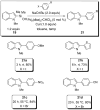
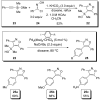
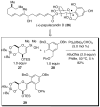
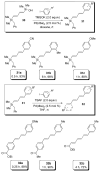
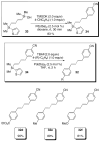
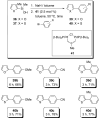
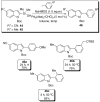
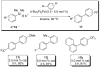
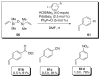
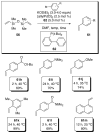
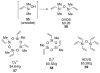
In the panoply of modern synthetic methods for forming carbon-carbon and carbon-heteroatom bonds, the transition metal-catalyzed cross-coupling of organometallic nucleophiles with organic electrophiles enjoys a preeminent status. The preparative utility of these reactions is, in large measure, a consequence of the wide variety of organometallic donors that have been conscripted into service. The most common of these reagents are organic derivatives of tin, boron, and zinc, which each possess unique advantages and shortcomings. Because of their low cost, low toxicity, and high chemical stability, organosilanes have emerged as viable alternatives to the conventional reagents in recent years. However, unlike the tin- and zinc-based reactions, which require no activation, or the boron-based reactions, which require only heating with mild bases, silicon-based cross-coupling reactions often require heating in the presence of a fluoride source; this has significantly hampered the widespread acceptance of organosilanes. To address the "fluoride problem", we have introduced a new paradigm for palladium-catalyzed, silicon-based cross-coupling reactions that employs organosilanols, a previously underutilized class of silicon reagents. The use of organosilanols either in the presence of Brønsted bases or as their silanolate salts represents a simple and mild alternative to the classic fluoride-based activation method. Organosilanols are easily available by many well-established methods for introducing carbon-silicon bonds onto alkenes, alkynes, and arenes and heteroarenes. Moreover, we have developed four different protocols for the generation of alkali metal salts of vinyl-, alkenyl-, alkynyl-, aryl-, and heteroarylsilanolates: (1) reversible deprotonation with weak Brønsted bases, (2) irreversible deprotonation with strong Brønsted bases, (3) isolation of the salts from irreversible deprotonation, and (4) silanolate exchange with disiloxanes. We have demonstrated the advantages of each of these methods for a number of different coupling classes. The defining feature of this new process is the formation of a covalently linked palladium silanolate species that facilitates the critical transmetalation step. We have verified the intermediacy of a critical species that contains the key Si-O-Pd linkage by its identification as the resting state in reaction mixtures, by X-ray analysis, and by demonstrating its competence in thermal cross-coupling with no additives. Our conclusions contradict the long-standing dogma that silicon-based cross-coupling reactions require the generation of a pentacoordinate siliconate prior to transmetalation. This revelation has opened a new vista for discovery of reactions that involve this critical process.
Figures








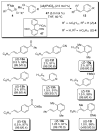
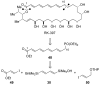

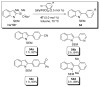
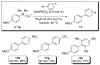

References
-
-
For reviews, see:de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Wiley-VCH; Weinheim: 2004. Negishi E, editor. Handbook of Organopalladium Chemistry. Wiley-Interscience; New York: 2002.
-
-
- Corriu RJP, Massé JP. Activation of Grignard Reagents by Transition-Metal Complexes. A New and Simple Synthesis of Trans-Stilbenes and Polyphenyls. J. Chem. Soc. Chem. Commun. 1972:144a.
- Tamao K, Sumitani K, Kumada M. Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes. J. Am. Chem. Soc. 1972;94:4374–4376.
-
-
For reviews on silicon-based cross-coupling, see:Hiyama T. Organosilicon Compounds in Cross-coupling Reactions. In: Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 1998. Chapter 10.Denmark SE, Sweis RF. Organosilicon Compounds in Cross-Coupling Reactions. In: de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Vol. 1. Wiley-VCH; Weinheim Germany: 2004. Chapter 4.Spivey AC, Gripton CJG, Hannah JP. Recent Advances in Group 14 Cross-Coupling: Si and Ge-Based Alternatives to the Stille Reaction. Curr. Org. Synth. 2004;1:211–226.
-
-
- Walsh R. Bond Dissociation Energy Values in Silicon-Containing Compounds and Some of Their Implications. Acc. Chem. Res. 1981;14:246–252.
-
- Damrauer R, Danahey SE. Preparation and NMR Studies of Pentacoordinated Silicon Anions. Organometallics. 1986;5:1490–1494.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

