NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs
- PMID: 18682241
- PMCID: PMC2758041
- DOI: 10.1016/j.stem.2008.07.001
NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs
Abstract
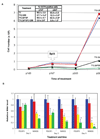
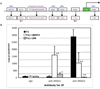
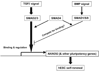
Self-renewal of human embryonic stem cells (ESCs) is promoted by FGF and TGFbeta/Activin signaling, and differentiation is promoted by BMP signaling, but how these signals regulate genes critical to the maintenance of pluripotency has been unclear. Using a defined medium, we show here that both TGFbeta and FGF signals synergize to inhibit BMP signaling; sustain expression of pluripotency-associated genes such as NANOG, OCT4, and SOX2; and promote long-term undifferentiated proliferation of human ESCs. We also show that both TGFbeta- and BMP-responsive SMADs can bind with the NANOG proximal promoter. NANOG promoter activity is enhanced by TGFbeta/Activin and FGF signaling and is decreased by BMP signaling. Mutation of putative SMAD binding elements reduces NANOG promoter activity to basal levels and makes NANOG unresponsive to BMP and TGFbeta signaling. These results suggest that direct binding of TGFbeta/Activin-responsive SMADs to the NANOG promoter plays an essential role in sustaining human ESC self-renewal.
Figures




Comment in
-
TGFbeta and SMADs talk to NANOG in human embryonic stem cells.Cell Stem Cell. 2008 Aug 7;3(2):127-8. doi: 10.1016/j.stem.2008.07.011. Cell Stem Cell. 2008. PMID: 18682233
References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. - PubMed
-
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. - PubMed
-
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2002;23:489–495. - PubMed
-
- Besser D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J Biol Chem. 2004;279:45076–45084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

