MSX2 stimulates chondrocyte maturation by controlling Ihh expression
- PMID: 18682398
- PMCID: PMC2662030
- DOI: 10.1074/jbc.M803681200
MSX2 stimulates chondrocyte maturation by controlling Ihh expression
Abstract
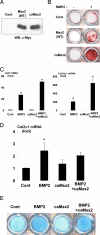
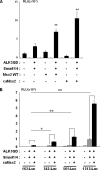
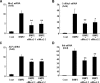
Several studies indicated that a homeobox gene, Msx2, is implicated in regulation of skeletal development by controlling enchondral ossification as well as membranous ossification. However, the molecular basis by which Msx2 conducts chondrogenesis is currently unclear. In this study, we examined the role of Msx2 in chondrocyte differentiation using mouse primary chondrocytes and embryonic metatarsal explants. Treatment with BMP2 up-regulated the expression of Msx2 mRNA along with chondrocyte differentiation in murine primary chondrocytes. Overexpression of wild-type Msx2 stimulated calcification of primary chondrocytes in the presence of BMP2. We also found that constitutively active Msx2 (caMsx2) enhanced BMP2-dependent calcification more efficiently than wild-type Msx2. Consistently, caMsx2 overexpression up-regulated the expression of alkaline phosphatase and collagen type X induced by BMP2. Furthermore, organ culture experiments using mouse embryonic metatarsals indicated that caMsx2 clearly stimulated the maturation of chondrocytes into the prehypertrophic and hypertrophic stages in the presence of BMP2. In contrast, knockdown of Msx2 inhibited maturation of primary chondrocytes. The stimulatory effect of Msx2 on chondrocyte maturation was enhanced by overexpression of Smad1 and Smad4 but inhibited by Smad6, an inhibitory Smad for BMP2 signaling. These data suggest that Msx2 requires BMP2/Smad signaling for its chondrogenic action. In addition, caMsx2 overexpression induced Ihh (Indian hedgehog) expression in mouse primary chondrocytes. Importantly, treatment with cyclopamine, a specific inhibitor for hedgehogs, blocked Msx2-induced chondrogenesis. Collectively, our results indicated that Msx2 promotes the maturation of chondrocytes, at least in part, through up-regulating Ihh expression.
Figures






References
-
- Lai, L. P., and Mitchell, J. (2005) J. Cell Biochem. 96 1163-1173 - PubMed
-
- Provot, S., and Schipani, E. (2005) Biochem. Biophys. Res. Commun. 328 658-665 - PubMed
-
- Kobayashi, T., and Kronenberg, H. (2005) Endocrinology 146 1012-1017 - PubMed
-
- Ryoo, H. M., Lee, M. H., and Kim, Y. J. (2006) Gene (Amst.) 366 51-57 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

