Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation
- PMID: 18682538
- PMCID: PMC2575905
- DOI: 10.1152/ajpendo.90314.2008
Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation
Abstract
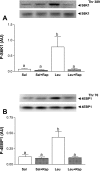
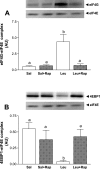
Skeletal muscle in the neonate grows at a rapid rate due in part to an enhanced sensitivity to the postprandial rise in amino acids, particularly leucine. To elucidate the molecular mechanism by which leucine stimulates protein synthesis in neonatal muscle, overnight-fasted 7-day-old piglets were treated with rapamycin [an inhibitor of mammalian target of rapamycin (mTOR) complex (mTORC)1] for 1 h and then infused with leucine for 1 h. Fractional rates of protein synthesis and activation of signaling components that lead to mRNA translation were determined in skeletal muscle. Rapamycin completely blocked leucine-induced muscle protein synthesis. Rapamycin markedly reduced raptor-mTOR association, an indicator of mTORC1 activation. Rapamycin blocked the leucine-induced phosphorylation of mTOR, S6 kinase 1 (S6K1), and eukaryotic initiation factor (eIF)4E-binding protein-1 (4E-BP1) and formation of the eIF4E.eIF4G complex and increased eIF4E.4E-BP1 complex abundance. Rapamycin had no effect on the association of mTOR with rictor, a crucial component for mTORC2 activation, or G protein beta-subunit-like protein (GbetaL), a component of mTORC1 and mTORC2. Neither leucine nor rapamycin affected the phosphorylation of AMP-activated protein kinase (AMPK), PKB, or tuberous sclerosis complex (TSC)2, signaling components that reside upstream of mTOR. Eukaryotic elongation factor (eEF)2 phosphorylation was not affected by leucine or rapamycin, although current dogma indicates that eEF2 phosphorylation is mTOR dependent. Together, these in vivo data suggest that leucine stimulates muscle protein synthesis in neonates by enhancing mTORC1 activation and its downstream effectors.
Figures





References
-
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000. - PubMed
-
- Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 134: 1704–1710, 2004. - PubMed
-
- Chien PF, Smith K, Watt PW, Scrimgeour CM, Taylor DJ, Rennie MJ. Protein turnover in the human fetus studied at term using stable isotope tracer amino acids. Am J Physiol Endocrinol Metab 265: E31–E35, 1993. - PubMed
-
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25: 6347–6360, 2006. - PubMed
-
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

