Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2
- PMID: 18682557
- PMCID: PMC2496887
- DOI: 10.1073/pnas.0800620105
Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2
Abstract
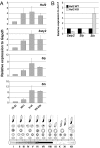
The mammalian Y chromosome is essential for spermatogenesis, which is characterized by sperm cell differentiation and chromatin condensation for acquisition of correct shape of the sperm. Deletions of the male-specific region of the mouse Y chromosome long arm (MSYq), harboring multiple copies of a few genes, lead to sperm head defects and impaired fertility. Using chromatin immunoprecipitation on promoter microarray (ChIP-chip) on mouse testis, we found a striking in vivo MSYq occupancy by heat shock factor 2 (HSF2), a transcription factor involved in spermatogenesis. HSF2 was also found to regulate the transcription of MSYq resident genes, whose transcriptional regulation has been unknown. Importantly, disruption of Hsf2 caused a similar phenotype as the 2/3 deletion of MSYq, i.e., altered expression of the multicopy genes and increased mild sperm head abnormalities. Consequently, aberrant levels of chromatin packing proteins and more frequent DNA fragmentation were detected, implying that HSF2 is required for correct chromatin organization in the sperm. Our findings define a physiological role for HSF2 in the regulation of MSYq resident genes and the quality of sperm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



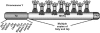
References
-
- Ellis PJ, Affara NA. Spermatogenesis and sex chromosome gene content: An evolutionary perspective. Hum Fertil (Cambridge) 2006;9:1–7. - PubMed
-
- Sarge KD, Park-Sarge O-K, Kirby JD, Mayo KE, Morimoto RI. Expression of heat shock factor 2 in mouse testis: Potential role as a regulator of heat-shock protein gene expression during spermatogenesis. Biol Reprod. 1994;50:1334–1343. - PubMed
-
- Alastalo T-P, et al. Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp Cell Res. 1998;240:16–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

