Detection of circulating tumour cells in peripheral blood with an automated scanning fluorescence microscope
- PMID: 18682708
- PMCID: PMC2528135
- DOI: 10.1038/sj.bjc.6604545
Detection of circulating tumour cells in peripheral blood with an automated scanning fluorescence microscope
Abstract
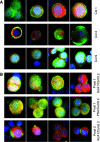
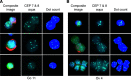
We have developed an automated, highly sensitive and specific method for identifying and enumerating circulating tumour cells (CTCs) in the blood. Blood samples from 10 prostate, 25 colorectal and 4 ovarian cancer patients were analysed. Eleven healthy donors and seven men with elevated serum prostate-specific antigen (PSA) levels but no evidence of malignancy served as controls. Spiking experiments with cancer cell lines were performed to estimate recovery yield. Isolation was performed either by density gradient centrifugation or by filtration, and the CTCs were labelled with monoclonal antibodies against cytokeratins 7/8 and either AUA1 (against EpCam) or anti-PSA. The slides were analysed with the Ikoniscope robotic fluorescence microscope imaging system. Spiking experiments showed that less than one epithelial cell per millilitre of blood could be detected, and that fluorescence in situ hybridisation (FISH) could identify chromosomal abnormalities in these cells. No positive cells were detected in the 11 healthy control samples. Circulating tumour cells were detected in 23 out of 25 colorectal, 10 out of 10 prostate and 4 out of 4 ovarian cancer patients. Five samples (three colorectal and two ovarian) were analysed by FISH for chromosomes 7 and 8 combined and all had significantly more than four dots per cell. We have demonstrated an Ikoniscope based relatively simple and rapid procedure for the clear-cut identification of CTCs. The method has considerable promise for screening, early detection of recurrence and evaluation of treatment response for a wide variety of carcinomas.
Figures

Similar articles
-
Method for semi-automated microscopy of filtration-enriched circulating tumor cells.BMC Cancer. 2016 Jul 14;16:477. doi: 10.1186/s12885-016-2461-4. BMC Cancer. 2016. PMID: 27417942 Free PMC article.
-
Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands.Int J Cancer. 2008 Oct 15;123(8):1968-73. doi: 10.1002/ijc.23717. Int J Cancer. 2008. PMID: 18661519 Free PMC article.
-
Development of an Automated and Sensitive Microfluidic Device for Capturing and Characterizing Circulating Tumor Cells (CTCs) from Clinical Blood Samples.PLoS One. 2016 Jan 25;11(1):e0147400. doi: 10.1371/journal.pone.0147400. eCollection 2016. PLoS One. 2016. PMID: 26808060 Free PMC article.
-
Detection of Gene Rearrangements in Circulating Tumor Cells: Examples of ALK-, ROS1-, RET-Rearrangements in Non-Small-Cell Lung Cancer and ERG-Rearrangements in Prostate Cancer.Adv Exp Med Biol. 2017;994:169-179. doi: 10.1007/978-3-319-55947-6_9. Adv Exp Med Biol. 2017. PMID: 28560674 Review.
-
Analysis of Circulating Tumor Cells in Prostate Cancer Patients at PSA Recurrence and Review of the Literature.Anticancer Res. 2016 Jun;36(6):2975-81. Anticancer Res. 2016. PMID: 27272813 Review.
Cited by
-
The potential for liquid biopsies in the precision medical treatment of breast cancer.Cancer Biol Med. 2016 Mar;13(1):19-40. doi: 10.28092/j.issn.2095-3941.2016.0007. Cancer Biol Med. 2016. PMID: 27144060 Free PMC article.
-
Research progress of CTC, ctDNA, and EVs in cancer liquid biopsy.Front Oncol. 2024 Jan 25;14:1303335. doi: 10.3389/fonc.2024.1303335. eCollection 2024. Front Oncol. 2024. PMID: 38333685 Free PMC article. Review.
-
Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes.Ann Clin Lab Sci. 2013 Summer;43(3):295-304. Ann Clin Lab Sci. 2013. PMID: 23884225 Free PMC article. Review.
-
A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells.Sensors (Basel). 2019 Apr 19;19(8):1879. doi: 10.3390/s19081879. Sensors (Basel). 2019. PMID: 31010258 Free PMC article.
-
Circulating Aneuploid Cells Detected in the Blood of Patients with Infectious Lung Diseases.Korean J Thorac Cardiovasc Surg. 2017 Apr;50(2):126-129. doi: 10.5090/kjtcs.2017.50.2.126. Epub 2017 Apr 5. Korean J Thorac Cardiovasc Surg. 2017. PMID: 28382274 Free PMC article.
References
-
- Ashworth TR (1869) A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aus Med J 14: 146–149
-
- Baran J, Pituch-Noworolska A, Krzeszowiak A, Wieckiewicz J, Stachura J, Pryjma J, Popiela T, Szczepanik A, Zembala M (1998) Detection of cancer cells in the blood by FACS sorting of CD45− cells. Int J Mol Med 1: 573–578 - PubMed
-
- Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsche HA, Islam R, Dawood S, Reuben JM, Kau SW, Lara JM, Krishnamurthy S, Ueno NT, Hortobagyi GN, Valero V (2007) Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 7: 471–479 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

