Protective immunity to Mycobacterium tuberculosis infection by chemokine and cytokine conditioned CFP-10 differentiated dendritic cells
- PMID: 18682728
- PMCID: PMC2478708
- DOI: 10.1371/journal.pone.0002869
Protective immunity to Mycobacterium tuberculosis infection by chemokine and cytokine conditioned CFP-10 differentiated dendritic cells
Abstract
Background: Dendritic cells (DCs) play major roles in mediating immune responses to mycobacteria. A crucial aspect of this is the priming of T cells via chemokines and cytokines. In this study we investigated the roles of chemokines RANTES and IP-10 in regulating protective responses from Mycobacterium tuberculosis (M. tb) 10 kDa Culture Filtrate Protein-10 (CFP-10) differentiated DCs (CFP10-DCs).
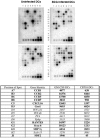
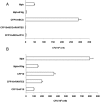
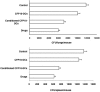
Methods and findings: Infection of CFP10-DCs with mycobacteria down-modulated RANTES and IP-10 levels. Pathway specific microarray analyses showed that in addition to RANTES and IP-10, mycobacteria infected CFP10-DCs showed reduced expression of many Th1 promoting chemokines and chemokine receptors. Importantly, T cells co-cultured with RANTES and IP-10 conditioned CFP10-DCs mediated killing of mycobacteria from infected macrophages. Similarly, T cells recruited by RANTES and IP-10 conditioned CFP10-DCs mediated significant killing of mycobacteria from infected macrophages. IFN-gamma treatment of CFP10-DCs restored RANTES and IP-10 levels and T cells activated by these DCs mediated significant killing of virulent M. tb inside macrophages. Adoptive transfer of either RANTES and IP-10 or IL-12 and IFN-gamma conditioned CFP10-DCs cleared an established M. tb infection in mice. The extent of clearance was similar to that obtained with drug treatment.
Conclusions: These results indicate that chemokine and cytokine secretion by DCs differentiated by M. tb antigens such as CFP-10 play major roles in regulating protective immune responses at sites of infection.
Conflict of interest statement
Figures
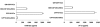







Similar articles
-
Differential live Mycobacterium tuberculosis-, M. bovis BCG-, recombinant ESAT6-, and culture filtrate protein 10-induced immunity in tuberculosis.Clin Vaccine Immunol. 2009 Jul;16(7):991-8. doi: 10.1128/CVI.00091-09. Epub 2009 May 13. Clin Vaccine Immunol. 2009. PMID: 19439524 Free PMC article.
-
Mycobacterium bovis and BCG induce different patterns of cytokine and chemokine production in dendritic cells and differentiation patterns in CD4+ T cells.Microbiology (Reading). 2013 Feb;159(Pt 2):366-379. doi: 10.1099/mic.0.058198-0. Epub 2012 Dec 6. Microbiology (Reading). 2013. PMID: 23223441
-
De Novo Fatty Acid Synthesis During Mycobacterial Infection Is a Prerequisite for the Function of Highly Proliferative T Cells, But Not for Dendritic Cells or Macrophages.Front Immunol. 2018 Apr 5;9:495. doi: 10.3389/fimmu.2018.00495. eCollection 2018. Front Immunol. 2018. PMID: 29675017 Free PMC article.
-
Mycobacterium tuberculosis and dendritic cells: recognition, activation and functional implications.Indian J Biochem Biophys. 2007 Oct;44(5):279-88. Indian J Biochem Biophys. 2007. PMID: 18341202 Review.
-
Distinct Strategies Employed by Dendritic Cells and Macrophages in Restricting Mycobacterium tuberculosis Infection: Different Philosophies but Same Desire.Int Rev Immunol. 2016 Sep 2;35(5):386-398. doi: 10.3109/08830185.2015.1015718. Epub 2015 Mar 20. Int Rev Immunol. 2016. PMID: 25793750 Review.
Cited by
-
Regulation of L-type Voltage Gated Calcium Channel CACNA1S in Macrophages upon Mycobacterium tuberculosis Infection.PLoS One. 2015 Apr 27;10(4):e0124263. doi: 10.1371/journal.pone.0124263. eCollection 2015. PLoS One. 2015. PMID: 25915405 Free PMC article.
-
Development of the first oligonucleotide microarray for global gene expression profiling in guinea pigs: defining the transcription signature of infectious diseases.BMC Genomics. 2012 Oct 2;13:520. doi: 10.1186/1471-2164-13-520. BMC Genomics. 2012. PMID: 23031549 Free PMC article.
-
Voltage gated calcium channels negatively regulate protective immunity to Mycobacterium tuberculosis.PLoS One. 2009;4(4):e5305. doi: 10.1371/journal.pone.0005305. Epub 2009 Apr 23. PLoS One. 2009. PMID: 19390594 Free PMC article.
-
Innate immune gene polymorphisms in tuberculosis.Infect Immun. 2012 Oct;80(10):3343-59. doi: 10.1128/IAI.00443-12. Epub 2012 Jul 23. Infect Immun. 2012. PMID: 22825450 Free PMC article. Review.
-
Acute immune response to Mycobacterium massiliense in C57BL/6 and BALB/c mice.Infect Immun. 2010 Apr;78(4):1571-81. doi: 10.1128/IAI.00731-09. Epub 2010 Feb 1. Infect Immun. 2010. PMID: 20123718 Free PMC article.
References
-
- World Health Organization. World Health Organization Fact Sheet, Geneva, Switzerland 2002.
-
- Steinman RM. Dendritic cells. In: William EPaul., editor. Fundamental Immunology. Lippincott-Raven Publishers, PA; 1999. pp. 547–573.
-
- Sousa CR. Dendritic cells as sensors of infection. Immunity. 2001;14:495–498. - PubMed
-
- Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates outcome of infection. J Immunol. 2005;175:3268–3272. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

