Plasticity of peptidergic innervation in healing rabbit medial collateral ligament
- PMID: 18682794
- PMCID: PMC2496588
Plasticity of peptidergic innervation in healing rabbit medial collateral ligament
Abstract
Background: Denervation substantially impairs healing of the medial collateral ligament (MCL). Because normal ligaments are sparsely innervated, we hypothesized that neuropeptide-containing neurons would sprout or proliferate after ligament transection, followed by later regression with healing, in a manner analogous to blood vessels.
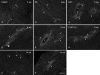
Methods: We transected the right MCL in 9 mature female New Zealand white rabbits and killed 3 rabbits at 2, 6 or 14 weeks. Alternate sets of 12-mm serial sections of healing MCL scars were examined by fluorescent immunohistochemistry for substance P (SP), calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY) and pan-neuronal marker PGP9.5.
Results: Normal MCLs had few peptidergic fibres located in the epiligament in a perivascular pattern. At 2 weeks, PGP9.5-, SP-and CGRP-positive fibres had increased in the epiligament adjacent to the injury. By 6 weeks, there were increases in CGRP-and PGP9.5-positive fibres in epiligament and scar, with similar but less marked increases in SP-positive fibres. At 14 weeks, there was notable regression of immunostained peptidergic nerve fibres in the scar.
Conclusion: This experiment shows evidence for a remarkable plasticity of ligament innervation after injury, supporting the idea that neuronal factors play a fundamental role in wound healing.
Contexte: La dénervation nuit considérablement à la guérison du ligament latéral interne (LLI). Parce que les ligaments normaux sont peu innervés, nous avons posé en hypothèse que des neurones contenant des neuropeptides feraient leur apparition ou se multiplieraient après une dissection transversale du ligament suivie d'une régression ultérieure avec la guérison, comme le font les vaisseaux sanguins.
Méthodes: Nous avons procédé à une dissection transversale du LLI droit chez 9 lapines blanches à maturité de la Nouvelle-Zélande et nous en avons tué 3 à 2, 6 ou 14 semaines. On a examiné par immunohistochimie fluorescente des séries alternantes de coupes de 12 mm de cicatrices LLI en guérison pour repérer la substance P (SP), le peptide lié au gène de la calcitonine (calcitonin gene-related peptide ou CGRP), le neuropeptide Y (NPY) et le marqueur pan-neuronal PGP9.5.
Résultats: Les LLI normaux contenaient peu de fibres peptidergiques situées dans l'épiligament disposées de façon périvasculaire. À 2 semaines, les fibres positives pour PGP9.5, SP et CGRP avaient augmenté dans l'épiligament adjacent au traumatisme. À 6 semaines, on a constaté des augmentations des fibres positives pour CGRP et PGP9.5 dans l'épiligament et la cicatrice et des augmentations semblables mais moins marquées des fibres positives pour la SP. À 14 semaines, on a constaté une régression notable des fibres nerveuses peptidergiques immunocolorées dans la cicatrice.
Conclusion: Cette expérience démontre une plasticité remarquable de l'innervation ligamentaire après une lésion, ce qui appuie le concept selon lequel des facteurs neuronaux jouent un rôle fondamental dans la guérison de la plaie.
Figures

Similar articles
-
Denervation impairs healing of the rabbit medial collateral ligament.J Orthop Res. 2002 Sep;20(5):990-5. doi: 10.1016/S0736-0266(02)00026-8. J Orthop Res. 2002. PMID: 12382964
-
Denervation alters mRNA levels of repair-associated genes in a rabbit medial collateral ligament injury model.J Orthop Res. 2006 Sep;24(9):1842-53. doi: 10.1002/jor.20219. J Orthop Res. 2006. PMID: 16865716
-
Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints.Anat Rec. 1997 May;248(1):29-39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. Anat Rec. 1997. PMID: 9143665
-
A role for calcitonin gene-related peptide in rabbit knee joint ligament healing.Can J Physiol Pharmacol. 2000 Jul;78(7):535-40. Can J Physiol Pharmacol. 2000. PMID: 10926159
-
Patterns of neuronal colocalisation of tyrosine hydroxylase, neuropeptide Y, vasoactive intestinal polypeptide, calcitonin gene-related peptide and substance P in human ureter.J Auton Nerv Syst. 1994 Aug;48(3):241-55. doi: 10.1016/0165-1838(94)90053-1. J Auton Nerv Syst. 1994. PMID: 7525686
Cited by
-
Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides.Auton Neurosci. 2020 Jan;223:102610. doi: 10.1016/j.autneu.2019.102610. Epub 2019 Nov 26. Auton Neurosci. 2020. PMID: 31790954 Free PMC article. Review.
-
Influence of Comorbidities: Neuropathy, Vasculopathy, and Diabetes on Healing Response Quality.Adv Wound Care (New Rochelle). 2013 Oct;2(8):410-421. doi: 10.1089/wound.2012.0437. Adv Wound Care (New Rochelle). 2013. PMID: 24688829 Free PMC article. Review.
-
The role of neuropeptides in cutaneous wound healing: a focus on mechanisms and neuropeptide-derived treatments.Front Bioeng Biotechnol. 2024 Oct 30;12:1494865. doi: 10.3389/fbioe.2024.1494865. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39539691 Free PMC article. Review.
-
Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: a model of myogenic orofacial pain.Mol Pain. 2010 Jul 15;6:40. doi: 10.1186/1744-8069-6-40. Mol Pain. 2010. PMID: 20633279 Free PMC article.
-
Neuronal regulation of tendon homoeostasis.Int J Exp Pathol. 2013 Aug;94(4):271-86. doi: 10.1111/iep.12028. Epub 2013 May 30. Int J Exp Pathol. 2013. PMID: 23718724 Free PMC article. Review.
References
-
- Clark RAF. Wound repair: overview and general considerations. In: Molecular and cellular biology of wound repair. New York: Plenum Press; 1996. p. 3-50.
-
- Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology 1997;37:133-52. - PubMed
-
- Chronwall BM, Zukowska Z. Neuropeptide Y, ubiquitous and elusive. Peptides 2004;25:359-63. - PubMed
-
- Kim LR, Whelpdale K, Zurowski M, et al. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen 1998;6:194-201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
