Protein crystals in Adenovirus type 5-infected cells: requirements for intranuclear crystallogenesis, structural and functional analysis
- PMID: 18682854
- PMCID: PMC2488365
- DOI: 10.1371/journal.pone.0002894
Protein crystals in Adenovirus type 5-infected cells: requirements for intranuclear crystallogenesis, structural and functional analysis
Abstract
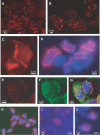
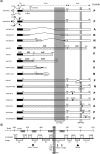
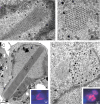
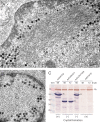
Intranuclear crystalline inclusions have been observed in the nucleus of epithelial cells infected with Adenovirus serotype 5 (Ad5) at late steps of the virus life cycle. Using immuno-electron microscopy and confocal microscopy of cells infected with various Ad5 recombinants modified in their penton base or fiber domains, we found that these inclusions represented crystals of penton capsomers, the heteromeric capsid protein formed of penton base and fiber subunits. The occurrence of protein crystals within the nucleus of infected cells required the integrity of the fiber knob and part of the shaft domain. In the knob domain, the region overlapping residues 489-492 in the FG loop was found to be essential for crystal formation. In the shaft, a large deletion of repeats 4 to 16 had no detrimental effect on crystal inclusions, whereas deletion of repeats 8 to 21 abolished crystal formation without altering the level of fiber protein expression. This suggested a crucial role of the five penultimate repeats in the crystallisation process. Chimeric pentons made of Ad5 penton base and fiber domains from different serotypes were analyzed with respect to crystal formation. No crystal was found when fiber consisted of shaft (S) from Ad5 and knob (K) from Ad3 (heterotypic S5-K3 fiber), but occurred with homotypic S3K3 fiber. However, less regular crystals were observed with homotypic S35-K35 fiber. TB5, a monoclonal antibody directed against the Ad5 fiber knob was found by immunofluorescence microscopy to react with high efficiency with the intranuclear protein crystals in situ. Data obtained with Ad fiber mutants indicated that the absence of crystalline inclusions correlated with a lower infectivity and/or lower yields of virus progeny, suggesting that the protein crystals might be involved in virion assembly. Thus, we propose that TB5 staining of Ad-infected 293 cells can be used as a prognostic assay for the viability and productivity of fiber-modified Ad5 vectors.
Conflict of interest statement
Figures








Similar articles
-
Immunoreactive domains and integrin-binding motifs in adenovirus penton base capsomer.Viral Immunol. 2000;13(3):353-71. doi: 10.1089/08828240050144671. Viral Immunol. 2000. PMID: 11016599
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
Deletion of the fiber gene induces the storage of hexon and penton base proteins in PML/Sp100-containing inclusions during adenovirus infection.Biol Cell. 1999 Nov;91(8):617-28. Biol Cell. 1999. PMID: 10629941
-
Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs.J Virol. 2005 Jan;79(2):1053-61. doi: 10.1128/JVI.79.2.1053-1061.2005. J Virol. 2005. PMID: 15613334 Free PMC article.
-
Novel partner proteins of adenovirus penton.Curr Top Microbiol Immunol. 2003;272:37-55. doi: 10.1007/978-3-662-05597-7_2. Curr Top Microbiol Immunol. 2003. PMID: 12747546 Review.
Cited by
-
Cell entry and trafficking of human adenovirus bound to blood factor X is determined by the fiber serotype and not hexon:heparan sulfate interaction.PLoS One. 2011;6(5):e18205. doi: 10.1371/journal.pone.0018205. Epub 2011 May 26. PLoS One. 2011. PMID: 21637339 Free PMC article.
-
Serotype 5 Adenovirus fiber (F7F41S) chimeric vectors incur packaging deficiencies when targeting peptides are inserted into Ad41 short fiber.Virology. 2009 Dec 5;395(1):10-20. doi: 10.1016/j.virol.2009.08.041. Epub 2009 Sep 25. Virology. 2009. PMID: 19782383 Free PMC article.
-
Tropism-modification strategies for targeted gene delivery using adenoviral vectors.Viruses. 2010 Oct;2(10):2290-2355. doi: 10.3390/v2102290. Epub 2010 Oct 13. Viruses. 2010. PMID: 21994621 Free PMC article.
-
PUMA gene delivery to synoviocytes reduces inflammation and degeneration of arthritic joints.Nat Commun. 2017 Jul 27;8(1):146. doi: 10.1038/s41467-017-00142-1. Nat Commun. 2017. PMID: 28747638 Free PMC article.
-
Visualizing viral protein structures in cells using genetic probes for correlated light and electron microscopy.Methods. 2015 Nov 15;90:39-48. doi: 10.1016/j.ymeth.2015.06.002. Epub 2015 Jun 9. Methods. 2015. PMID: 26066760 Free PMC article. Review.
References
-
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–2604. - PubMed
-
- Novelli A, Boulanger P. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991;185:365–376. - PubMed
-
- Novelli A, Boulanger PA. Assembly of adenovirus type 2 fiber synthesized in cell-free translation system. J Biol Chem. 1991;266:9299–9303. - PubMed

