Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva
- PMID: 18684712
- PMCID: PMC2652274
- DOI: 10.1074/jbc.M801681200
Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva
Abstract
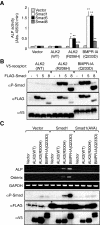
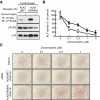
Fibrodysplasia ossificans progressiva (FOP) is a rare autosomal dominant disorder characterized by congenital malformation of the great toes and by progressive heterotopic bone formation in muscle tissue. Recently, a mutation involving a single amino acid substitution in a bone morphogenetic protein (BMP) type I receptor, ALK2, was identified in patients with FOP. We report here that the identical mutation, R206H, was observed in 19 Japanese patients with sporadic FOP. This mutant receptor, ALK2(R206H), activates BMP signaling without ligand binding. Moreover, expression of Smad1 and Smad5 was up-regulated in response to muscular injury. ALK2(R206H) with Smad1 or Smad5 induced osteoblastic differentiation that could be inhibited by Smad7 or dorsomorphin. Taken together, these findings suggest that the heterotopic bone formation in FOP may be induced by a constitutively activated BMP receptor signaling through Smad1 or Smad5. Gene transfer of Smad7 or inhibition of type I receptors with dorsomorphin may represent strategies for blocking the activity induced by ALK2(R206H) in FOP.
Figures


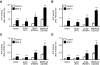

References
-
- Cohen, R. B., Hahn, G. V., Tabas, J. A., Peeper, J., Levitz, C. L., Sando, A., Sando, N., Zasloff, M., and Kaplan, F. S. (1993) J. Bone Jt. Surg. Am. 75 215-219 - PubMed
-
- Kaplan, F. S., McCluskey, W., Hahn, G., Tabas, J. A., Muenke, M., and Zasloff, M. A. (1993) J. Bone Jt. Surg. Am. 75 1214-1220 - PubMed
-
- Kaplan, F. S., Tabas, J. A., Gannon, F. H., Finkel, G., Hahn, G. V., and Zasloff, M. A. (1993) J. Bone Jt. Surg. Am. 75 220-230 - PubMed
-
- Kaplan, F. S., Glaser, D. L., Shore, E. M., Pignolo, R. J., Xu, M., Zhang, Y., Senitzer, D., Forman, S. J., and Emerson, S. G. (2007) J. Bone Jt. Surg. Am. 89 347-357 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

