The glandular stem/progenitor cell niche in airway development and repair
- PMID: 18684717
- PMCID: PMC2645260
- DOI: 10.1513/pats.200801-003AW
The glandular stem/progenitor cell niche in airway development and repair
Abstract
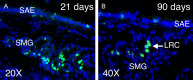
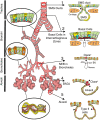
Airway submucosal glands (SMGs) are major secretory structures that lie beneath the epithelium of the cartilaginous airway. These glands are believed to play important roles in normal lung function and airway innate immunity by secreting antibacterial factors, mucus, and fluid into the airway lumen. Recent studies have suggested that SMGs may additionally serve as a protective niche for adult epithelial stem/progenitor cells of the proximal airways. As in the case of other adult stem cell niches, SMGs are believed to provide the localized environmental signals required to both maintain and mobilize stem/progenitor cells, in the setting of normal cellular turnover or injury. Aberrant proliferation and differentiation of glandular stem/progenitor cells may be associated with several hypersecretory lung diseases, including chronic bronchitis, asthma, and cystic fibrosis. To better understand the molecular mechanisms that regulate the specification and proliferation of glandular stem/progenitor cells in lung diseases associated with SMG hypertrophy and hyperplasia, researchers have begun to search for the molecular signals and cell types responsible for establishing the glandular stem/progenitor cell niche, and to dissect how these determinants of the niche change in the setting of proximal airway injury and repair. Such studies have revealed certain similarities between stem/progenitor cell niches of the distal conducting airways and the SMGs of the proximal airways.
Figures
References
-
- Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis 1983;128:S14–S20. - PubMed
-
- Tom-Moy M, Basbaum CB, Nadel JA. Localization and release of lysozyme from ferret trachea: effects of adrenergic and cholinergic drugs. Cell Tissue Res 1983;228:549–562. - PubMed
-
- Widdicombe JH, Bastacky SJ, Wu DX, Lee CY. Regulation of depth and composition of airway surface liquid. Eur Respir J 1997;10:2892–2897. - PubMed
-
- Nadel JA, Davis B. Parasympathetic and sympathetic regulation of secretion from submucosal glands in airways. Fed Proc 1980;39:3075–3079. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
