Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT)
- PMID: 18684743
- PMCID: PMC2663711
- DOI: 10.1136/ard.2008.092767
Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT)
Abstract
Objective: To evaluate the long-term effectiveness and tolerability of adalimumab in the treatment of psoriatic arthritis (PsA).
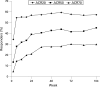
Methods: Patients with PsA who completed a 24-week, double-blind study of adalimumab versus placebo were eligible to enroll in an open-label extension study and receive adalimumab 40 mg subcutaneously every other week for up to an additional 120 weeks. At the time of this analysis, available efficacy evaluations throughout 2 years of treatment (n = 245) included American College of Rheumatology (ACR) 20%, 50% and 70% improvement scores, measures of joint disease and skin disease, disability and quality of life; modified total Sharp scores (mTSS) were available for 2.75 years of treatment for patients who received adalimumab in the 24-week study.
Results: After 24 weeks of double-blind treatment, the mean change in mTSS was -0.2 for the adalimumab group (N = 144) and 1.0 for the placebo group (N = 152; p<0.001), and outcomes for all individual ACR component variables were significantly improved in adalimumab compared with placebo-treated patients. Compared with 24-week responses, inhibition of radiographic progression and improvements in joint disease were maintained in most patients during long-term, open-label adalimumab treatment. Also, improvements in skin disease were maintained, with >20% of patients achieving the strict criterion of psoriasis area and severity index 100. The nature and frequency of adverse events during long-term adalimumab treatment were consistent with the safety profile during short-term treatment.
Conclusions: The clinical and radiographic efficacy of adalimumab demonstrated during short-term treatment was sustained during long-term treatment. Adalimumab has a favourable risk-benefit profile in patients with PsA.
Trial registration number: NCT00195689.
Conflict of interest statement
Figures

References
-
- Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mørk C, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13 - PubMed
-
- Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA): an analysis of 220 patients. Q J Med 1987;62:127–41 - PubMed
-
- Gladman DD, Brockbank J. Psoriatic arthritis. Expert Opin Investig Drugs 2000;9:1511–22 - PubMed
-
- Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

