Functions of Varicella-zoster virus ORF23 capsid protein in viral replication and the pathogenesis of skin infection
- PMID: 18684828
- PMCID: PMC2566272
- DOI: 10.1128/JVI.01890-07
Functions of Varicella-zoster virus ORF23 capsid protein in viral replication and the pathogenesis of skin infection
Abstract
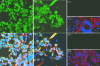
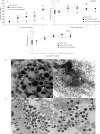
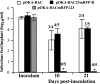
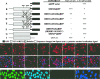
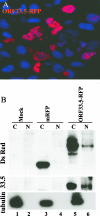
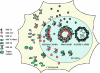
The assembly of herpesvirus capsids is a complex process involving interactions of multiple proteins in the cytoplasm and in the nucleus. Based on comparative genome analyses, varicella-zoster virus (VZV) open reading frame 23 (ORF23) encodes a conserved capsid protein, referred to as VP26 (UL35) in other alphaherpesviruses. Mutagenesis using a VZV bacterial artificial chromosome system showed that ORF23 was dispensable for replication in vitro. However, the absence of ORF23 disrupted capsid assembly in a melanoma cell line. Expression of ORF23 as a red fluorescent protein (RFP) fusion protein appeared to have a dominant negative effect on replication that was rescued by ORF23 expression from a nonnative site in the VZV genome. In contrast to its VP26 homolog, ORF23 has an intrinsic nuclear localization capacity that was mapped to an SRSRVV motif at residues 229 to 234 in the extreme C terminus of ORF23. In addition, coexpression with ORF23 resulted in nuclear import of the major capsid protein, ORF40. VZV ORF33.5 also translocated ORF40, which may provide a redundant mechanism in vitro but appears insufficient to overcome the dominant negative effect of the monomeric RFP-ORF23 (mRFP23) fusion protein. ORF23 was required for VZV infection of human skin xenografts, indicating that ORF33.5 does not compensate for lack of ORF23 in vivo. These observations suggest a model of VZV capsid assembly in which nuclear transport of the major capsid protein and associated proteins requires ORF23 during VZV replication in the human host. If so, ORF23 expression could be a target for a novel antiviral drug against VZV.
Figures
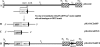






References
-
- Cohen, J. I., S. E. Straus, and A. M. Arvin. 2007. Varicella-zoster virus, p. 2773-2818. In D. H. Knipe, D. E. Griffin, R. A. Lamb, S. E. Straus, P. M. Howley, M. A. Martin, and B. Roizman (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

