Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2
- PMID: 18684832
- PMCID: PMC2566291
- DOI: 10.1128/JVI.01133-08
Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2
Abstract
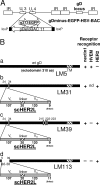
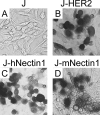
A novel frontier in the treatment of tumors that are difficult to treat is oncolytic virotherapy, in which a replication-competent virus selectively infects and destroys tumor cells. Herpes simplex virus (HSV) represents a particularly attractive system. Effective retargeting to tumor-specific receptors has been achieved by insertion in gD of heterologous ligands. Previously, our laboratory generated an HSV retargeted to human epidermal growth factor receptor 2 (HER2), a receptor overexpressed in about one-third of mammary tumors and in some ovarian tumors. HER2 overexpression correlates with increased metastaticity and poor prognosis. Because HER2 has no natural ligand, the inserted ligand was a single-chain antibody to HER2. The objective of this work was to genetically engineer an HSV that selectively targets the HER2-expressing tumor cells and that has lost the ability to enter cells through the natural gD receptors, HVEM and nectin1. Detargeting from nectin1 was attempted by two different strategies, point mutations and insertion of the single-chain antibody at a site in gD different from previously described sites of insertion. We report that point mutations at gD amino acids 34, 215, 222, and 223 failed to generate a nectin1-detargeted HSV. An HSV simultaneously detargeted from nectin1 and HVEM and retargeted to HER2 was successfully engineered by moving the site of single-chain antibody insertion at residue 39, i.e., in front of the nectin1-interacting surface and not lateral to it, and by deleting amino acid residues 6 to 38. The resulting recombinant, R-LM113, entered cells and spread from cell to cell solely via HER2.
Figures




References
-
- Advani, S. J., R. R. Weichselbaum, R. J. Whitley, and B. Roizman. 2002. Friendly fire: redirecting herpes simplex virus-1 for therapeutic applications. Clin. Microbiol. Infect. 8551-563. - PubMed
-
- Aghi, M., and R. L. Martuza. 2005. Oncolytic viral therapies—the clinical experience. Oncogene 247802-7816. - PubMed
-
- Andreansky, S., L. Soroceanu, E. R. Flotte, J. Chou, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1997. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 571502-1509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

