CD20, CD3, and CD40 ligand microclusters segregate three-dimensionally in vivo at B-cell-T-cell immunological synapses after viral immunity in primate brain
- PMID: 18684835
- PMCID: PMC2566292
- DOI: 10.1128/JVI.01326-08
CD20, CD3, and CD40 ligand microclusters segregate three-dimensionally in vivo at B-cell-T-cell immunological synapses after viral immunity in primate brain
Abstract
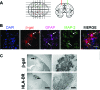
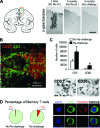
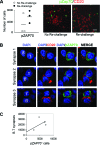
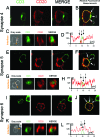
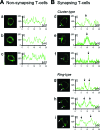
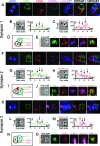
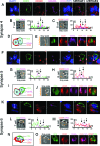
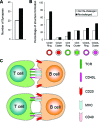
The clearance of virally infected cells from the brain is mediated by T cells that engage antigen-presenting cells to form supramolecular activation clusters at the immunological synapse. However, after clearance, the T cells persist at the infection site and remain activated locally. In the present work the long-term interactions of immune cells in brains of monkeys were imaged in situ 9 months after the viral inoculation. After viral immunity, the persistent infiltration of T cells and B cells was observed at the infection sites. T cells showed evidence of T-cell receptor signaling as a result of contacts with B cells. Three-dimensional analysis of B-cell-T-cell synapses showed clusters of CD3 in T cells and the segregation of CD20 in B cells, involving the recruitment of CD40 ligand at the interface. These results demonstrate that immunological synapses between B cells and T cells forming three-dimensional microclusters occur in vivo in the central nervous system and suggest that these interactions may be involved in the lymphocyte activation after viral immunity at the original infection site.
Figures






References
-
- Allen, C. D., T. Okada, H. L. Tang, and J. G. Cyster. 2007. Imaging of germinal center selection events during affinity maturation. Science 315528-531. - PubMed
-
- Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, C. van Kooten, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12881-922. - PubMed
-
- Barcia, C., M. Jimenez-Dalmaroni, K. M. Kroeger, M. Puntel, A. J. Rapaport, D. Larocque, G. D. King, S. A. Johnson, C. Liu, W. Xiong, M. Candolfi, S. Mondkar, P. Ng, D. Palmer, M. G. Castro, and P. R. Lowenstein. 2007. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol. Ther. 152154-2163. - PMC - PubMed
-
- Barcia, C., A. Sanchez Bahillo, E. Fernandez-Villalba, V. Bautista, Y. P. M. Poza, A. Fernandez-Barreiro, E. C. Hirsch, and M. T. Herrero. 2004. Evidence of active microglia in substantia nigra pars compacta of Parkinsonian monkeys 1 year after MPTP exposure. Glia 46402-409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

