The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells
- PMID: 18684887
- PMCID: PMC2576149
- DOI: 10.1152/ajprenal.00559.2007
The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells
Abstract
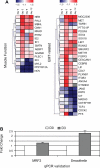
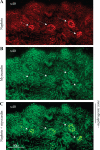
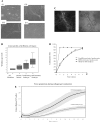
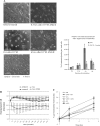
The glomerular podocyte is a highly specialized cell, with the ability to ultrafilter blood and support glomerular capillary pressures. However, little is known about either the genetic programs leading to this functionality or the final phenotype. We approached this question utilizing a human conditionally immortalized cell line, which differentiates from a proliferating epithelial phenotype to a differentiated form. We profiled gene expression during several time points during differentiation and grouped the regulated genes into major functional categories. A novel category of genes that was upregulated during differentiation was of smooth muscle-related molecules. We further examined the smooth muscle phenotype and showed that podocytes consistently express the differentiated smooth muscle markers smoothelin and calponin and the specific transcription factor myocardin, both in vitro and in vivo. The contractile contribution of the podocyte to the glomerular capillary is controversial. We demonstrated using two novel techniques that podocytes contract vigorously in vitro when differentiated and in real time were able to demonstrate that angiotensin II treatment decreases monolayer resistance, morphologically correlating with enhanced contractility. We conclude that the mature podocyte in vitro possesses functional apparatus of contractile smooth muscle cells, with potential implications for its in vivo ability to regulate glomerular dynamic and permeability characteristics.
Figures



References
-
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs 169: 1–11, 2001. - PubMed
-
- Andrews PM, Coffey AK. Cytoplasmic contractile elements in glomerular cells. Fed Proc 42: 3046–3052, 1983. - PubMed
-
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol 30: 35–44, 2001. - PubMed
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

