Radical-free biology of oxidative stress
- PMID: 18684987
- PMCID: PMC2575825
- DOI: 10.1152/ajpcell.00283.2008
Radical-free biology of oxidative stress
Abstract
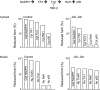
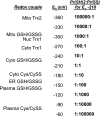
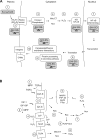
Free radical-induced macromolecular damage has been studied extensively as a mechanism of oxidative stress, but large-scale intervention trials with free radical scavenging antioxidant supplements show little benefit in humans. The present review summarizes data supporting a complementary hypothesis for oxidative stress in disease that can occur without free radicals. This hypothesis, which is termed the "redox hypothesis," is that oxidative stress occurs as a consequence of disruption of thiol redox circuits, which normally function in cell signaling and physiological regulation. The redox states of thiol systems are sensitive to two-electron oxidants and controlled by the thioredoxins (Trx), glutathione (GSH), and cysteine (Cys). Trx and GSH systems are maintained under stable, but nonequilibrium conditions, due to a continuous oxidation of cell thiols at a rate of about 0.5% of the total thiol pool per minute. Redox-sensitive thiols are critical for signal transduction (e.g., H-Ras, PTP-1B), transcription factor binding to DNA (e.g., Nrf-2, nuclear factor-kappaB), receptor activation (e.g., alphaIIbbeta3 integrin in platelet activation), and other processes. Nonradical oxidants, including peroxides, aldehydes, quinones, and epoxides, are generated enzymatically from both endogenous and exogenous precursors and do not require free radicals as intermediates to oxidize or modify these thiols. Because of the nonequilibrium conditions in the thiol pathways, aberrant generation of nonradical oxidants at rates comparable to normal oxidation may be sufficient to disrupt function. Considerable opportunity exists to elucidate specific thiol control pathways and develop interventional strategies to restore normal redox control and protect against oxidative stress in aging and age-related disease.
Figures






References
-
- Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990. - PubMed
-
- Abramson JLJD, Bremner JD, Quyyumi AA, Goldberg J, Vaccarino V. Oxidative stress, as indicated by the redox states of plasma thiol/disulfide couples, is associated with myocardial perfusion defects in men without clinical CAD (Abstract). JACC 45: 401A, 2005.
-
- Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem 279: 29857–29862, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

