Connexin26 deafness associated mutations show altered permeability to large cationic molecules
- PMID: 18684989
- PMCID: PMC2575827
- DOI: 10.1152/ajpcell.00008.2008
Connexin26 deafness associated mutations show altered permeability to large cationic molecules
Abstract
Intercellular communication is important for cochlear homeostasis because connexin26 (Cx26) mutations are the leading cause of hereditary deafness. Gap junctions formed by different connexins have unique selectivity to large molecules, so compensating for the loss of one isoform can be challenging in the case of disease causing mutations. We compared the properties of Cx26 mutants T8M and N206S with wild-type channels in transfected cells using dual whole cell voltage clamp and dye flux experiments. Wild-type and mutant channels demonstrated comparable ionic coupling, and their average unitary conductance was approximately 106 and approximately 60 pS in 120 mM K(+)-aspartate(-) and TEA(+)-aspartate(-) solution, respectively, documenting their equivalent permeability to K(+) and TEA(+). Comparison of cAMP, Lucifer Yellow (LY), and ethidium bromide (EtBr) transfer revealed differences in selectivity for larger anionic and cationic tracers. cAMP and LY permeability to wild-type and mutant channels was similar, whereas the transfer of EtBr through mutant channels was greatly reduced compared with wild-type junctions. Altered permeability of Cx26 to large cationic molecules suggests an essential role for biochemical coupling in cochlear homeostasis.
Figures






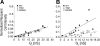
References
-
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem 281: 7994–8009, 2006. - PubMed
-
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D'Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun 305: 1024–1033, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

