Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus
- PMID: 18685033
- PMCID: PMC2647017
- DOI: 10.1523/JNEUROSCI.1288-08.2008
Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus
Abstract
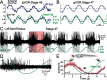
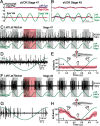
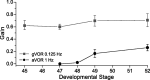
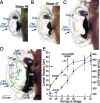
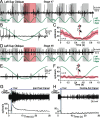
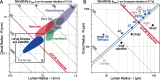
Semicircular canals have been sensors of angular acceleration for 450 million years. This vertebrate adaptation enhances survival by implementing postural and visual stabilization during motion in a three-dimensional environment. We used an integrated neuroethological approach in larval Xenopus to demonstrate that semicircular canal dimensions, and not the function of other elements, determines the onset of angular acceleration detection. Before angular vestibuloocular function in either the vertical or horizontal planes, at stages 47 and 48, respectively, each individual component of the vestibuloocular system was shown to be operational: extraocular muscles could be activated, central neural pathways were complete, and canal hair cells were capable of evoking graded responses. For Xenopus, a minimum semicircular canal lumen radius of 60 microm was necessary to permit endolymph displacement sufficient for sensor function at peak accelerations of 400 degrees /s(2). An intra-animal comparison demonstrated that this size is reached in the vertical canals earlier in development than in the horizontal canals, corresponding to the earlier onset of vertical canal-activated ocular motor behavior. Because size constitutes a biophysical threshold for canal-evoked behavior in other vertebrates, such as zebrafish, we suggest that the semicircular canal lumen and canal circuit radius are limiting the onset of vestibular function in all small vertebrates. Given that the onset of gravitoinertial acceleration detection precedes angular acceleration detection by up to 10 d in Xenopus, these results question how the known precise spatial patterning of utricular and canal afferents in adults is achieved during development.
Figures



References
-
- Angelaki DE. Eyes on target: what neurons must do for the vestibuloocular reflex during linear motion. J Neurophysiol. 2004;92:20–35. - PubMed
-
- Baker R, Gilland E. The evolution of hindbrain visual and vestibular innovations responsible for oculomotor function. In: Bloedel JR, Ebner TJ, Wise SP, editors. The acquisition of motor behavior in vertebrates. Cambridge, MA: MIT; 1996. pp. 29–55.
-
- Beck JC, Baker R. Static and dynamic measurement of otolithic VOR during the absence of canal function in larval zebrafish. Soc Neurosci Abstr. 2005;31 391.16.
-
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. J Neurophysiol. 2004a;92:3546–3561. - PubMed
-
- Beck JC, Gilland E, Baker R, Tank DW. Instrumentation for measuring oculomotor performance and plasticity in larval organisms. Methods Cell Biol. 2004b;76:385–413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
