Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor
- PMID: 18685038
- PMCID: PMC6670768
- DOI: 10.1523/JNEUROSCI.1006-08.2008
Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor
Abstract
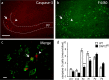
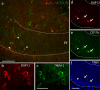
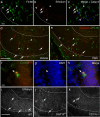
In several brain regions, microglia actively promote neuronal apoptosis during development. However, molecular actors leading microglia to trigger death remain mostly unknown. Here, we show that, in the developing hippocampus, apoptotic neurons are contacted by microglia expressing both the integrin CD11b and the immunoreceptor DAP12. We demonstrate that developmental apoptosis decreases in mice deficient for CD11b or DAP12. In addition, function-blocking antibodies directed against CD11b decrease neuronal death when injected into wild-type neonates, but have no effect when injected into DAP12-deficient littermates. This demonstrates that DAP12 and CD11b act in converging pathways to induce neuronal death. Finally, we show that DAP12 and CD11b control the production of microglial superoxide ions, which kill the neurons. Thus, our data show that the process of developmental neuronal death triggered by microglia is similar to the elimination of pathogenic cells by the innate immune cells.
Figures

References
-
- Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. - PubMed
-
- Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. - PubMed
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
