Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice
- PMID: 18685102
- PMCID: PMC2497459
- DOI: 10.1073/pnas.0805361105
Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice
Abstract
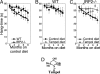
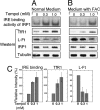
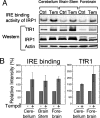
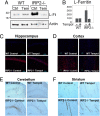
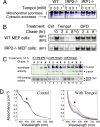
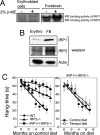
In mammals, two homologous cytosolic regulatory proteins, iron regulatory protein 1 (also known as IRP1 and Aco1) and iron regulatory protein 2 (also known as IRP2 and Ireb2), sense cytosolic iron levels and posttranscriptionally regulate iron metabolism genes, including transferrin receptor 1 (TfR1) and ferritin H and L subunits, by binding to iron-responsive elements (IREs) within target transcripts. Mice that lack IRP2 develop microcytic anemia and neurodegeneration associated with functional cellular iron depletion caused by low TfR1 and high ferritin expression. IRP1 knockout (IRP1(-/-)) animals do not significantly misregulate iron metabolism, partly because IRP1 is an iron-sulfur protein that functions mainly as a cytosolic aconitase in mammalian tissues and IRP2 activity increases to compensate for loss of the IRE binding form of IRP1. The neurodegenerative disease of IRP2(-/-) animals progresses slowly as the animals age. In this study, we fed IRP2(-/-) mice a diet supplemented with a stable nitroxide, Tempol, and showed that the progression of neuromuscular impairment was markedly attenuated. In cell lines derived from IRP2(-/-) animals, and in the cerebellum, brainstem, and forebrain of animals maintained on the Tempol diet, IRP1 was converted from a cytosolic aconitase to an IRE binding protein that stabilized the TfR1 transcript and repressed ferritin synthesis. We suggest that Tempol protected IRP2(-/-) mice by disassembling the cytosolic iron-sulfur cluster of IRP1 and activating IRE binding activity, which stabilized the TfR1 transcript, repressed ferritin synthesis, and partially restored normal cellular iron homeostasis in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. - PubMed
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: Molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. - PubMed
-
- Dupuy J, et al. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006;14:129–139. - PubMed
-
- Walden WE, et al. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

