Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia
- PMID: 18685564
- PMCID: PMC2762405
- DOI: 10.1038/clpt.2008.154
Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia
Abstract
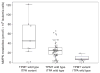
The influence of genetic polymorphism in inosine triphosphate pyrophosphatase (ITPA) on thiopurine-induced adverse events has not been investigated in the context of combination chemotherapy for acute lymphoblastic leukemia (ALL). This study investigated the effects of a common ITPA variant allele (rs41320251) on mercaptopurine metabolism and toxicity during treatment of children with ALL. Significantly higher concentrations of methyl mercaptopurine nucleotides were found in patients with the nonfunctional ITPA allele. Moreover, there was a significantly higher probability of severe febrile neutropenia in patients with a variant ITPA allele among patients whose dose of mercaptopurine had been adjusted for TPMT genotype. In a cohort of patients whose mercaptopurine dose was not adjusted for TPMT phenotype, the TPMT genotype had a greater effect than the ITPA genotype. In conclusion, genetic polymorphism of ITPA is a significant determinant of mercaptopurine metabolism and of severe febrile neutropenia, after combination chemotherapy for ALL in which mercaptopurine doses are individualized on the basis of TPMT genotype.
Conflict of interest statement
WE Evans is a co-inventor on a patent awarded for the molecular diagnosis of the major
Figures





References
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. - PubMed
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. - PubMed
-
- Rivera GK, et al. Unexpectedly severe toxicity from intensive early treatment of childhood lymphoblastic leukemia. J Clin Oncol. 1985;3:201–206. - PubMed
-
- Evans WE, Relling MV. Moving towards individualized medicine with pharmacogenomics. Nature. 2004;429:464–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

