Accelerating drug development using biomarkers: a case study with sitagliptin, a novel DPP4 inhibitor for type 2 diabetes
- PMID: 18686043
- PMCID: PMC2751391
- DOI: 10.1208/s12248-008-9041-8
Accelerating drug development using biomarkers: a case study with sitagliptin, a novel DPP4 inhibitor for type 2 diabetes
Abstract
The leveraged use of biomarkers presents an opportunity in understanding target engagement and disease impact while accelerating drug development. For effective integration in drug development, it is essential for biomarkers to aid in the elucidation of mechanisms of action and disease progression. The recent years have witnessed significant progress in biomarker selection, validation, and qualification, while enabling surrogate and clinical endpoint qualification and application. Biomarkers play a central role in target validation for novel mechanisms. They also play a central role in the learning/confirming paradigm, particularly when utilized in concert with pharmacokinetic/pharmacodynamic modeling. Clearly, these attributes make biomarker integration attractive for scientific and regulatory applications to new drug development. In this review, applications of proximal, or target engagement, and distal, or disease-related, biomarkers are highlighted using the example of the recent development of sitagliptin for type 2 diabetes, wherein elucidation of target engagement and disease-related biomarkers significantly accelerated sitagliptin drug development. Importantly, use of biomarkers as tools facilitated design of clinical efficacy trials while streamlining dose focus and optimization, the net impact of which reduced overall cycle time to filing as compared to the industry average.
Figures


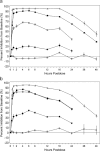

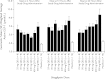
References
-
- R. Krishna. Introduction to dose optimization. In R. Krishna (ed.), Dose optimization in drug development, Taylor and Francis, v161, 2006, pp. 1–13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

