Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease
- PMID: 18686734
- PMCID: PMC2629972
- DOI: 10.2147/copd.s2089
Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease
Abstract
Since the early 1960s, a compelling body of evidence has accumulated to show that proteinases play critical roles in airspace enlargement in chronic obstructive pulmonary disease (COPD). However, until recently the causative enzymes and their exact roles in pathologic processes in COPD have not been clear. Recent studies of gene-targeted mice in murine models of COPD have confirmed roles for proteinases not only in airspace enlargement, but also in airway pathologies in COPD. These studies have also shed light on the specific proteinases involved in COPD pathogenesis, and the mechanisms by which these proteinases injure the lung. They have also identified important interactions between different classes of proteinases, and between proteinases and other molecules that amplify lung inflammation and injury. This review will discuss the biology of proteinases and the mechanisms by which they contribute to the pathogenesis of COPD. In addition, I will discuss the potential of proteinase inhibitors and anti-inflammatory drugs as new treatment strategies for COPD patients.
Figures
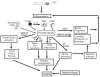



References
-
- Abboud RT, Fera T, Richter A, et al. Acute effect of smoking on the functional activity of alpha1-protease inhibitor in bronchoalveolar lavage fluid. ARRD. 1985;131:79–85. - PubMed
-
- Abboud RT, Ofulue AF, Sansores RH, et al. Relationship of alveolar macrophage plasminogen activator and elastase activities to lung function and CT evidence of emphysema. Chest. 1998;113:1257–63. - PubMed
-
- Amitani R, Wilson R, Rutmen A, et al. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. - PubMed
-
- Amour A, Slocombe PM, Webster A, et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

