Nuclear import of Avian Sarcoma Virus integrase is facilitated by host cell factors
- PMID: 18687138
- PMCID: PMC2527327
- DOI: 10.1186/1742-4690-5-73
Nuclear import of Avian Sarcoma Virus integrase is facilitated by host cell factors
Abstract
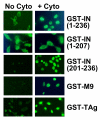
Background: Integration of retroviral DNA into the host cell genome is an obligatory step in the virus life cycle. In previous reports we identified a sequence (amino acids 201-236) in the linker region between the catalytic core and C-terminal domains of the avian sarcoma virus (ASV) integrase protein that functions as a transferable nuclear localization signal (NLS) in mammalian cells. The sequence is distinct from all known NLSs but, like many, contains basic residues that are essential for activity.
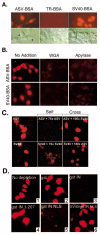
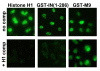
Results: Our present studies with digitonin-permeabilized HeLa cells show that nuclear import mediated by the NLS of ASV integrase is an active, saturable, and ATP-dependent process. As expected for transport through nuclear pore complexes, import is blocked by treatment of cells with wheat germ agglutinin. We also show that import of ASV integrase requires soluble cellular factors but does not depend on binding the classical adapter Importin-alpha. Results from competition studies indicate that ASV integrase relies on one or more of the soluble components that mediate transport of the linker histone H1.
Conclusion: These results are consistent with a role for ASV integrase and cytoplasmic cellular factors in the nuclear import of its viral DNA substrate, and lay the foundation for identification of host cell components that mediate this reaction.
Figures

References
-
- Suzuki Y, Craigie R. The road to chromatin - nuclear entry of retroviruses. Nat Rev Microbiol. 2007;5:187–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

