Engineering a high-affinity allosteric binding site for divalent cations in kainate receptors
- PMID: 18687343
- PMCID: PMC2629458
- DOI: 10.1016/j.neuropharm.2008.07.013
Engineering a high-affinity allosteric binding site for divalent cations in kainate receptors
Abstract
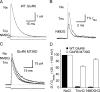
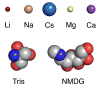
Kainate receptors are allosterically regulated by sodium ions. Removal of Na+ from the extracellular solution, or replacement of Na+ by larger monovalent cations, inhibits kainate receptor activity. Sodium binds at a negatively charged cavity in the extracellular neurotransmitter binding domain that is capped by a small hydrophobic residue. Prior work revealed that introduction of aspartic acid at this site strongly reduces GluK2 sensitivity to monovalent cations of different size. We found that the GluK2 M739D mutant is also insensitive to substitution of Na+ by the large organic cations Tris and NMDG. Because these are excluded from the Na+ binding site by steric hindrance, we investigated the possibility that divalent cations can substitute for Na+. We show that in Na+ free solutions with low concentrations of Ca2+ and Mg2+ the GluK2 M739D mutant is inhibited by EDTA; that divalent ions in the micromolar concentration range act as positive allosteric modulators; and that the chemistry of the mutant cation binding site is typical of Ca2+ and Mg2+ binding sites found in protein crystal structures. Hence, the apparent insensitivity of the M739D mutant to monovalent cations is due to the adventitious allosteric effects of divalent ions at physiological concentrations and below.
Figures

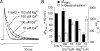

References
-
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. - PubMed
-
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
-
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

