Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice
- PMID: 18687778
- PMCID: PMC2734496
- DOI: 10.1210/en.2008-0775
Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice
Abstract
Energy homeostasis involves central nervous system integration of afferent inputs that coordinately regulate food intake and energy expenditure. Here, we report that adult homozygous TNFalpha converting enzyme (TACE)-deficient mice exhibit one of the most dramatic examples of hypermetabolism yet reported in a rodent system. Because this effect is not matched by increased food intake, mice lacking TACE exhibit a lean phenotype. In the hypothalamus of these mice, neurons in the arcuate nucleus exhibit intact responses to reduced fat mass and low circulating leptin levels, suggesting that defects in other components of the energy homeostasis system explain the phenotype of Tace(DeltaZn/DeltaZn) mice. Elevated levels of uncoupling protein-1 in brown adipose tissue from Tace(DeltaZn/DeltaZn) mice when compared with weight-matched controls suggest that deficient TACE activity is linked to increased sympathetic outflow. These findings collectively identify a novel and potentially important role for TACE in energy homeostasis.
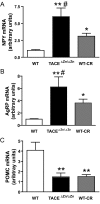
Figures
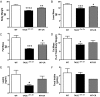
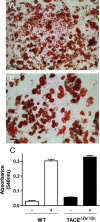
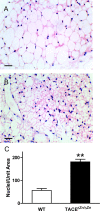
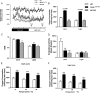

References
-
- Seals DF, Courtneidge SA 2003 The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 17:7–30 - PubMed
-
- Blobel CP 2005 ADAMS: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6:32–43 - PubMed
-
- Ohtsu H, Dempsey PJ, Eguchi S 2006 ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 291:C1–C10 - PubMed
-
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP 1997 A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729–733 - PubMed
-
- Moss ML, Jin SL, Becherer JD, Bickett DM, Burkhart W, Chen WJ, Hassler D, Leesnitzer MT, McGeehan G, Milla M, Moyer M, Rocque W, Seaton T, Schoenen F, Warner J, Willard D 1997 Structural features and biochemical properties of TNF-α converting enzyme (TACE). J Neuroimmunol 72:127–129 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

