A photoactive carotenoid protein acting as light intensity sensor
- PMID: 18687902
- PMCID: PMC2575289
- DOI: 10.1073/pnas.0804636105
A photoactive carotenoid protein acting as light intensity sensor
Abstract
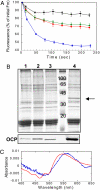
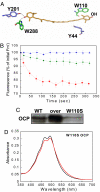
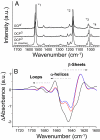
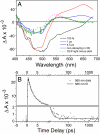
Intense sunlight is dangerous for photosynthetic organisms. Cyanobacteria, like plants, protect themselves from light-induced stress by dissipating excess absorbed energy as heat. Recently, it was discovered that a soluble orange carotenoid protein, the OCP, is essential for this photoprotective mechanism. Here we show that the OCP is also a member of the family of photoactive proteins; it is a unique example of a photoactive protein containing a carotenoid as the photoresponsive chromophore. Upon illumination with blue-green light, the OCP undergoes a reversible transformation from its dark stable orange form to a red "active" form. The red form is essential for the induction of the photoprotective mechanism. The illumination induces structural changes affecting both the carotenoid and the protein. Thus, the OCP is a photoactive protein that senses light intensity and triggers photoprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
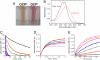

References
-
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. - PubMed
-
- Niyogi K. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Mol Biol. 1999;50:333–359. - PubMed
-
- Demming-Adams B, Adams WW., III Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2179–2153. - PubMed
-
- Holt N, Zigmantas D, Valkunas L, Li X-P, Niyogi K, Fleming G. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

