Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis
- PMID: 18687984
- PMCID: PMC2582000
- DOI: 10.1182/blood-2008-02-140814
Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis
Abstract
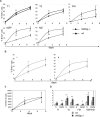
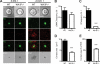
To more precisely identify the B-cell phenotype in Wiskott-Aldrich syndrome (WAS), we used 3 distinct murine in vivo models to define the cell intrinsic requirements for WAS protein (WASp) in central versus peripheral B-cell development. Whereas WASp is dispensable for early bone marrow B-cell development, WASp deficiency results in a marked reduction in each of the major mature peripheral B-cell subsets, exerting the greatest impact on marginal zone and B1a B cells. Using in vivo bromodeoxyuridine labeling and in vitro functional assays, we show that these deficits reflect altered peripheral homeostasis, partially resulting from an impairment in integrin function, rather than a developmental defect. Consistent with these observations, we also show that: (1) WASp expression levels increase with cell maturity, peaking in those subsets exhibiting the greatest sensitivity to WASp deficiency; (2) WASp(+) murine B cells exhibit a marked selective advantage beginning at the late transitional B-cell stage; and (3) a similar in vivo selective advantage is manifest by mature WASp(+) human B cells. Together, our data provide a better understanding of the clinical phenotype of WAS and suggest that gene therapy might be a useful approach to rescue altered B-cell homeostasis in this disease.
Figures





Comment in
-
WASp stings mature lymphocytes.Blood. 2008 Nov 15;112(10):3921-2. doi: 10.1182/blood-2008-09-176685. Blood. 2008. PMID: 18988873 Free PMC article.
References
-
- Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–885. - PubMed
-
- Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. - PubMed
-
- Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. quiz 739. - PubMed
-
- Thrasher AJ. WASp in immune-system organization and function. Nat Rev Immunol. 2002;2:635–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

