An antimetastatic role for decorin in breast cancer
- PMID: 18688028
- PMCID: PMC2527080
- DOI: 10.2353/ajpath.2008.080275
An antimetastatic role for decorin in breast cancer
Abstract
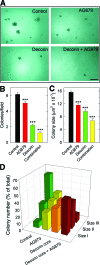
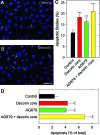
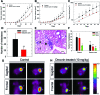
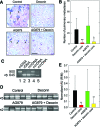
Decorin, a member of the small leucine-rich proteoglycan gene family, down-regulates members of the ErbB receptor tyrosine kinase family and attenuates their signaling, leading to growth inhibition. We investigated the effects of decorin on the growth of ErbB2-overexpressing mammary carcinoma cells in comparison with AG879, an established ErbB2 kinase inhibitor. Cell proliferation and anchorage-independent growth assays showed that decorin was a potent inhibitor of breast cancer cell growth and a pro-apoptotic agent. When decorin and AG879 were used in combination, the inhibitory effect was synergistic in proliferation assays but only additive in both colony formation and apoptosis assays. Active recombinant human decorin protein core, AG879, or a combination of both was administered systemically to mice bearing orthotopic mammary carcinoma xenografts. Primary tumor growth and metabolism were reduced by approximately 50% by both decorin and AG879. However, no synergism was observed in vivo. Decorin specifically targeted the tumor cells and caused a significant reduction of ErbB2 levels in the tumor xenografts. Most importantly, systemic delivery of decorin prevented metastatic spreading to the lungs, as detected by novel species-specific DNA detection and quantitative assays. In contrast, AG879 failed to have any effect. Our data support a role for decorin as a powerful and effective therapeutic agent against breast cancer due to its inhibition of both primary tumor growth and metastatic spreading.
Figures



References
-
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. - PubMed
-
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. - PubMed
-
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. - PubMed
-
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2003;19:249–255. - PubMed
-
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

