The role of B-cells and IgM antibodies in parasitemia, anemia, and VSG switching in Trypanosoma brucei-infected mice
- PMID: 18688274
- PMCID: PMC2483930
- DOI: 10.1371/journal.ppat.1000122
The role of B-cells and IgM antibodies in parasitemia, anemia, and VSG switching in Trypanosoma brucei-infected mice
Abstract
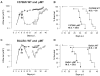
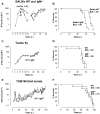
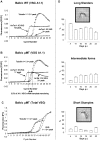
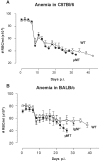
African trypanosomes are extracellular parasitic protozoa, predominantly transmitted by the bite of the haematophagic tsetse fly. The main mechanism considered to mediate parasitemia control in a mammalian host is the continuous interaction between antibodies and the parasite surface, covered by variant-specific surface glycoproteins. Early experimental studies have shown that B-cell responses can be strongly protective but are limited by their VSG-specificity. We have used B-cell (microMT) and IgM-deficient (IgM(-/-)) mice to investigate the role of B-cells and IgM antibodies in parasitemia control and the in vivo induction of trypanosomiasis-associated anemia. These infection studies revealed that that the initial setting of peak levels of parasitemia in Trypanosoma brucei-infected microMT and IgM(-/-) mice occurred independent of the presence of B-cells. However, B-cells helped to periodically reduce circulating parasites levels and were required for long term survival, while IgM antibodies played only a limited role in this process. Infection-associated anemia, hypothesized to be mediated by B-cell responses, was induced during infection in microMT mice as well as in IgM(-/-) mice, and as such occurred independently from the infection-induced host antibody response. Antigenic variation, the main immune evasion mechanism of African trypanosomes, occurred independently from host antibody responses against the parasite's ever-changing antigenic glycoprotein coat. Collectively, these results demonstrated that in murine experimental T. brucei trypanosomiasis, B-cells were crucial for periodic peak parasitemia clearance, whereas parasite-induced IgM antibodies played only a limited role in the outcome of the infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. - PubMed
-
- WHO Control and surveillance of African trypanosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1998;881:I–VI. 1–114. - PubMed
-
- Van den Bossche P. Some general aspects of the distribution and epidemiology of bovine trypanosomosis in southern Africa. Int J Parasitol. 2001;31:592–598. - PubMed
-
- van Meirvenne N. Paris ; London: Springer; 1999. Biological diagnosis of human African trypanosomiasis. Progress in human African trypanosomiasis, sleeping sickness. pp. 235–252.
-
- Vanhamme L, Pays E, McCulloch R, Barry JD. An update on antigenic variation in African trypanosomes. Trends Parasitol. 2001;17:338–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

