Human cytomegalovirus UL18 utilizes US6 for evading the NK and T-cell responses
- PMID: 18688275
- PMCID: PMC2483941
- DOI: 10.1371/journal.ppat.1000123
Human cytomegalovirus UL18 utilizes US6 for evading the NK and T-cell responses
Abstract
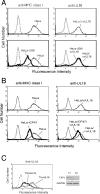
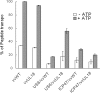
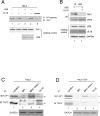
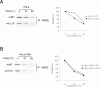
Human cytomegalovirus (HCMV) US6 glycoprotein inhibits TAP function, resulting in down-regulation of MHC class I molecules at the cell surface. Cells lacking MHC class I molecules are susceptible to NK cell lysis. HCMV expresses UL18, a MHC class I homolog that functions as a surrogate to prevent host cell lysis. Despite a high level of sequence and structural homology between UL18 and MHC class I molecules, surface expression of MHC class I, but not UL18, is down regulated by US6. Here, we describe a mechanism of action by which HCMV UL18 avoids attack by the self-derived TAP inhibitor US6. UL18 abrogates US6 inhibition of ATP binding by TAP and, thereby, restores TAP-mediated peptide translocation. In addition, UL18 together with US6 interferes with the physical association between MHC class I molecules and TAP that is required for optimal peptide loading. Thus, regardless of the recovery of TAP function, surface expression of MHC class I molecules remains decreased. UL18 represents a unique immune evasion protein that has evolved to evade both the NK and the T cell immune responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The MHC class I homolog of human cytomegalovirus is resistant to down-regulation mediated by the unique short region protein (US)2, US3, US6, and US11 gene products.J Immunol. 2002 Apr 1;168(7):3464-9. doi: 10.4049/jimmunol.168.7.3464. J Immunol. 2002. PMID: 11907106
-
Increased expression of leukocyte Ig-like receptor-1 and activating role of UL18 in the response to cytomegalovirus infection.J Immunol. 2007 Mar 15;178(6):3536-43. doi: 10.4049/jimmunol.178.6.3536. J Immunol. 2007. PMID: 17339449
-
Human cytomegalovirus UL40 signal peptide regulates cell surface expression of the NK cell ligands HLA-E and gpUL18.J Immunol. 2012 Mar 15;188(6):2794-804. doi: 10.4049/jimmunol.1102068. Epub 2012 Feb 15. J Immunol. 2012. PMID: 22345649 Free PMC article.
-
Immune modulation by the human cytomegalovirus-encoded molecule UL18, a mystery yet to be solved.J Immunol. 2008 Jan 1;180(1):19-24. doi: 10.4049/jimmunol.180.1.19. J Immunol. 2008. PMID: 18096997 Review.
-
Human leukocyte antigen E in human cytomegalovirus infection: friend or foe?Acta Biochim Biophys Sin (Shanghai). 2012 Jul;44(7):551-4. doi: 10.1093/abbs/gms032. Epub 2012 May 9. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22576308 Review.
Cited by
-
The polymorphism at residue 156 determines the HLA-B*35 restricted peptide repertoire during HCMV infection.Immunogenetics. 2018 Nov;70(10):639-646. doi: 10.1007/s00251-018-1077-z. Epub 2018 Aug 21. Immunogenetics. 2018. PMID: 30128813 Free PMC article.
-
Human Cytomegalovirus Infection Increases Both Antibody- and Non-Antibody-Dependent Cellular Reactivity by Natural Killer Cells.Transplant Direct. 2017 Nov 20;3(12):e335. doi: 10.1097/TXD.0000000000000750. eCollection 2017 Dec. Transplant Direct. 2017. PMID: 29536036 Free PMC article.
-
RNA-binding protein CPEB1 remodels host and viral RNA landscapes.Nat Struct Mol Biol. 2016 Dec;23(12):1101-1110. doi: 10.1038/nsmb.3310. Epub 2016 Oct 24. Nat Struct Mol Biol. 2016. PMID: 27775709 Free PMC article.
-
Battle between Host Immune Cellular Responses and HCMV Immune Evasion.Int J Mol Sci. 2019 Jul 24;20(15):3626. doi: 10.3390/ijms20153626. Int J Mol Sci. 2019. PMID: 31344940 Free PMC article. Review.
-
Herpesvirus Evasion of Natural Killer Cells.J Virol. 2018 May 14;92(11):e02105-17. doi: 10.1128/JVI.02105-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29540598 Free PMC article. Review.
References
-
- Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12(Suppl. 7):S701–710. - PubMed
-
- Wattre P, Dewilde A, Lobert PE. [Current status of human cytomegalovirus disease]. Rev Med Interne. 1995;16:354–367. - PubMed
-
- Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. - PubMed
-
- Elliott T, Williams A. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol Rev. 2005;207:89–99. - PubMed
-
- Park B, Lee S, Kim E, Cho K, Riddell SR, et al. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell. 2006;127:369–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

