Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial
- PMID: 18689435
- PMCID: PMC2525447
- DOI: 10.1093/brain/awn173
Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial
Abstract
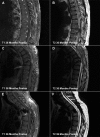
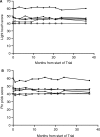
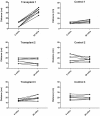
Olfactory ensheathing cells show promise in preclinical animal models as a cell transplantation therapy for repair of the injured spinal cord. This is a report of a clinical trial of autologous transplantation of olfactory ensheathing cells into the spinal cord in six patients with complete, thoracic paraplegia. We previously reported on the methods of surgery and transplantation and the safety aspects of the trial 1 year after transplantation. Here we address the overall design of the trial and the safety of the procedure, assessed during a period of 3 years following the transplantation surgery. All patients were assessed at entry into the trial and regularly during the period of the trial. Clinical assessments included medical, psychosocial, radiological and neurological, as well as specialized tests of neurological and functional deficits (standard American Spinal Injury Association and Functional Independence Measure assessments). Quantitative test included neurophysiological tests of sensory and motor function below the level of injury. The trial was a Phase I/IIa design whose main aim was to test the feasibility and safety of transplantation of autologous olfactory ensheathing cells into the injured spinal cord in human paraplegia. The design included a control group who did not receive surgery, otherwise closely matched to the transplant recipient group. This group acted as a control for the assessors, who were blind to the treatment status of the patients. The control group also provided the opportunity for preliminary assessment of the efficacy of the transplantation. There were no adverse findings 3 years after autologous transplantation of olfactory ensheathing cells into spinal cords injured at least 2 years prior to transplantation. The magnetic resonance images (MRIs) at 3 years showed no change from preoperative MRIs or intervening MRIs at 1 and 2 years, with no evidence of any tumour of introduced cells and no development of post-traumatic syringomyelia or other adverse radiological findings. There were no significant functional changes in any patients and no neuropathic pain. In one transplant recipient, there was an improvement over 3 segments in light touch and pin prick sensitivity bilaterally, anteriorly and posteriorly. We conclude that transplantation of autologous olfactory ensheathing cells into the injured spinal cord is feasible and is safe up to 3 years of post-implantation, however, this conclusion should be considered preliminary because of the small number of trial patients.
Figures
Comment in
-
Ready for human spinal cord repair?Brain. 2008 Sep;131(Pt 9):2240-2. doi: 10.1093/brain/awn185. Brain. 2008. PMID: 18728095 Review. No abstract available.
-
Clinical trials for the treatment of spinal cord injury: cervical and lumbar enlargements versus thoracic area.Brain. 2009 Jul;132(Pt 7):e115; author reply e116. doi: 10.1093/brain/awn282. Epub 2008 Nov 20. Brain. 2009. PMID: 19022857 Review. No abstract available.
References
-
- Beazley LD, Rodger J, Chen P, Tee LB, Stirling RV, Taylor AL, et al. Training on a visual task improves the outcome of optic nerve regeneration. J Neurotrauma. 2003;20:1263–70. - PubMed
-
- Bianco JI, Perry C, Harkin DG, Mackay-Sim A, Feron F. Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia. 2004;45:111–23. - PubMed
-
- Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med. 2007;30:421–40. - PMC - PubMed
-
- Campbell J, Kendall M. Investigating the suitability of the clinical outcome variables scale (COVS) as a mobility outcome measure in spinal cord injury rehabilitation. Physiotherapy Canada. 2003;55:135–44.
-
- Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. The spinal cord independence measure (SCIM): sensitivity to functional changes in subgroups of spinal cord lesion patients. Spinal Cord. 2001;39:97–100. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

