LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis
- PMID: 18689444
- PMCID: PMC2556827
- DOI: 10.1104/pp.108.126342
LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis
Abstract
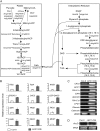
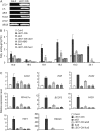
In plants, fatty acids are de novo synthesized predominantly in plastids from acetyl-coenzyme A. Although fatty acid biosynthesis has been biochemically well studied, little is known about the regulatory mechanisms of the pathway. Here, we show that overexpression of the Arabidopsis (Arabidopsis thaliana) LEAFY COTYLEDON1 (LEC1) gene causes globally increased expression of fatty acid biosynthetic genes, which are involved in key reactions of condensation, chain elongation, and desaturation of fatty acid biosynthesis. In the plastidial fatty acid synthetic pathway, over 58% of known enzyme-coding genes are up-regulated in LEC1-overexpressing transgenic plants, including those encoding three subunits of acetyl-coenzyme A carboxylase, a key enzyme controlling the fatty acid biosynthesis flux. Moreover, genes involved in glycolysis and lipid accumulation are also up-regulated. Consistent with these results, levels of major fatty acid species and lipids were substantially increased in the transgenic plants. Genetic analysis indicates that the LEC1 function is partially dependent on ABSCISIC ACID INSENSITIVE3, FUSCA3, and WRINKLED1 in the regulation of fatty acid biosynthesis. Moreover, a similar phenotype was observed in transgenic Arabidopsis plants overexpressing two LEC1-like genes of Brassica napus. These results suggest that LEC1 and LEC1-like genes act as key regulators to coordinate the expression of fatty acid biosynthetic genes, thereby representing promising targets for genetic improvement of oil production plants.
Figures



References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40 151–160
-
- Baud S, Guyon V, Kronenberger J, Wuilleme S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33 75–86 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

