Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes
- PMID: 18689454
- PMCID: PMC2576378
- DOI: 10.1529/biophysj.108.128728
Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes
Abstract
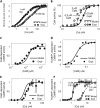
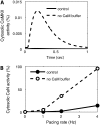
Calmodulin (CaM) mediates Ca-dependent regulation of numerous pathways in the heart, including CaM-dependent kinase (CaMKII) and calcineurin (CaN), yet the local Ca(2+) signals responsible for their selective activation are unclear. To assess when and where CaM, CaMKII, and CaN may be activated in the cardiac myocyte, we integrated new mechanistic computational models of CaM, CaMKII, and CaN with the Shannon-Bers model of excitation-contraction coupling in the rabbit ventricular myocyte. These models are validated with independent in vitro data. In the intact myocyte, model simulations predict that CaM is highly activated in the dyadic cleft during each beat, but not appreciably in the cytosol. CaMKII-delta(C) was almost insensitive to cytosolic Ca due to relatively low CaM affinity. Dyadic cleft CaMKII exhibits dynamic frequency-dependent responses to Ca, yet autophosphorylates only when local phosphatases are suppressed. In contrast, dyadic cleft CaN in beating myocytes is predicted to be constitutively active, whereas the extremely high affinity of CaN for CaM allows gradual integration of small cytosolic CaM signals. Reversing CaM affinities for CaMKII and CaN also reverses their characteristic local responses. Deactivation of both CaMKII and CaN seems dominated by Ca dissociation from the complex (versus Ca-CaM dissociation from the target). In summary, the different affinities of CaM for CaMKII and CaN determine their sensitivity to local Ca signals in cardiac myocytes.
Figures







References
-
- Bush, E. W., D. B. Hood, P. J. Papst, J. A. Chapo, W. Minobe, M. R. Bristow, E. N. Olson, and T. A. McKinsey. 2006. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 281:33487–33496. - PubMed
-
- Persechini, A., and P. M. Stemmer. 2002. Calmodulin is a limiting factor in the cell. Trends Cardiovasc. Med. 12:32–37. - PubMed
-
- Berridge, M. J. 2006. Calcium microdomains: organization and function. Cell Calcium. 40:405–412. - PubMed
-
- Quintana, A. R., D. Wang, J. E. Forbes, and M. N. Waxham. 2005. Kinetics of calmodulin binding to calcineurin. Biochem. Biophys. Res. Commun. 334:674–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

