Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension
- PMID: 18689495
- PMCID: PMC2593516
- DOI: 10.1152/ajpheart.00596.2008
Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension
Abstract
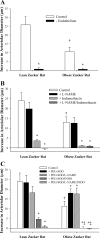
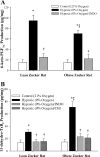
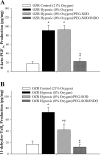
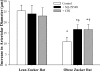
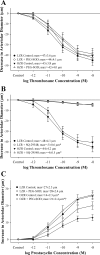
This study determined if altered vascular prostacyclin (PGI(2)) and/or thromboxane A(2) (TxA(2)) production with reduced Po(2) contributes to impaired hypoxic dilation of skeletal muscle resistance arterioles of obese Zucker rats (OZRs) versus lean Zucker rats (LZRs). Mechanical responses were assessed in isolated gracilis muscle arterioles following reductions in Po(2) under control conditions and following pharmacological interventions inhibiting arachidonic acid metabolism and nitric oxide synthase and alleviating elevated vascular oxidant stress. The production of arachidonic acid metabolites was assessed using pooled arteries from OZRs and LZRs in response to reduced Po(2). Hypoxic dilation, endothelium-dependent in both strains, was attenuated in OZRs versus LZRs. Nitric oxide synthase inhibition had no significant impact on hypoxic dilation in either strain. Cyclooxygenase inhibition dramatically reduced hypoxic dilation in LZRs and abolished responses in OZRs. Treatment of arterioles from OZRs with polyethylene glycol-superoxide dismutase improved hypoxic dilation, and this improvement was entirely cyclooxygenase dependent. Vascular PGI(2) production with reduced Po(2) was similar between strains, although TxA(2) production was increased in OZRs, a difference that was attenuated by treatment of vessels from OZRs with polyethylene glycol-superoxide dismutase. Both blockade of PGH(2)/TxA(2) receptors and inhibition of thromboxane synthase increased hypoxic dilation in OZR arterioles. These results suggest that a contributing mechanism underlying impaired hypoxic dilation of skeletal muscle arterioles of OZRs may be an increased vascular production of TxA(2), which competes against the vasodilator influences of PGI(2). These results also suggest that the elevated vascular oxidant stress inherent in metabolic syndrome may contribute to the increased vascular TxA(2) production and may blunt vascular sensitivity to PGI(2).
Figures
Similar articles
-
Insulin resistance and impaired functional vasodilation in obese Zucker rats.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1658-66. doi: 10.1152/ajpheart.01206.2007. Epub 2008 Feb 22. Am J Physiol Heart Circ Physiol. 2008. PMID: 18296567
-
Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats.Am J Physiol Heart Circ Physiol. 2001 Sep;281(3):H1304-11. doi: 10.1152/ajpheart.2001.281.3.H1304. Am J Physiol Heart Circ Physiol. 2001. PMID: 11514301
-
Impaired dilation of skeletal muscle microvessels to reduced oxygen tension in diabetic obese Zucker rats.Am J Physiol Heart Circ Physiol. 2001 Oct;281(4):H1568-74. doi: 10.1152/ajpheart.2001.281.4.H1568. Am J Physiol Heart Circ Physiol. 2001. PMID: 11557545
-
Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn.Pharmacol Rev. 2012 Jul;64(3):540-82. doi: 10.1124/pr.111.004770. Epub 2012 Jun 7. Pharmacol Rev. 2012. PMID: 22679221 Free PMC article. Review.
-
The thromboxane synthase and receptor signaling pathway in cancer: an emerging paradigm in cancer progression and metastasis.Cancer Metastasis Rev. 2011 Dec;30(3-4):397-408. doi: 10.1007/s10555-011-9297-9. Cancer Metastasis Rev. 2011. PMID: 22037941 Free PMC article. Review.
Cited by
-
Hypertension in metabolic syndrome: vascular pathophysiology.Int J Hypertens. 2013;2013:230868. doi: 10.1155/2013/230868. Epub 2013 Mar 20. Int J Hypertens. 2013. PMID: 23573411 Free PMC article.
-
Regulation of vascular smooth muscle tone by adipose-derived contracting factor.PLoS One. 2013 Nov 11;8(11):e79245. doi: 10.1371/journal.pone.0079245. eCollection 2013. PLoS One. 2013. PMID: 24244459 Free PMC article.
-
Effects of impaired microvascular flow regulation on metabolism-perfusion matching and organ function.Microcirculation. 2021 Apr;28(3):e12673. doi: 10.1111/micc.12673. Epub 2020 Dec 21. Microcirculation. 2021. PMID: 33236393 Free PMC article. Review.
-
The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an update.Basic Res Cardiol. 2012 Jan;107(1):237. doi: 10.1007/s00395-011-0237-1. Epub 2011 Dec 22. Basic Res Cardiol. 2012. PMID: 22189563 Free PMC article. Review.
-
The ex vivo isolated skeletal microvessel preparation for investigation of vascular reactivity.J Vis Exp. 2012 Apr 28;(62):3674. doi: 10.3791/3674. J Vis Exp. 2012. PMID: 22565845 Free PMC article.
References
-
- Asghar M, Monjok E, Kouamou G, Ohia SE, Bagchi D, Lokhandwala MF. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol Cell Biochem 304: 93–99, 2007. - PubMed
-
- Bachschmid M, Schildknecht S, Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun 338: 536–542, 2005. - PubMed
-
- Bachschmid M, Thurau S, Zou MH, Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J 17: 914–916, 2003. - PubMed
-
- Bouvet C, de Chantemèle EB, Guihot AL, Vessières E, Bocquet A, Dumont O, Jardel A, Loufrani L, Moreau P, Henrion D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 50: 248–254, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

