The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly
- PMID: 18689507
- PMCID: PMC2565960
- DOI: 10.1128/AEM.00741-08
The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly
Abstract
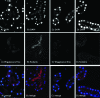
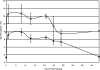
Tsetse flies (Diptera: Glossinidae) are vectors for trypanosome parasites, the agents of the deadly sleeping sickness disease in Africa. Tsetse also harbor two maternally transmitted enteric mutualist endosymbionts: the primary intracellular obligate Wigglesworthia glossinidia and the secondary commensal Sodalis glossinidius. Both endosymbionts are transmitted to the intrauterine progeny through the milk gland secretions of the viviparous female. We administered various antibiotics either continuously by per os supplementation of the host blood meal diet or discretely by hemocoelic injections into fertile females in an effort to selectively eliminate the symbionts to study their individual functions. A symbiont-specific PCR amplification assay and fluorescence in situ hybridization analysis were used to evaluate symbiont infection outcomes. Tetracycline and rifampin treatments eliminated all tsetse symbionts but reduced the fecundity of the treated females. Ampicillin treatments did not affect the intracellular Wigglesworthia localized in the bacteriome organ and retained female fecundity. The resulting progeny of ampicillin-treated females, however, lacked Wigglesworthia but still harbored the commensal Sodalis. Our results confirm the presence of two physiologically distinct Wigglesworthia populations: the bacteriome-localized Wigglesworthia involved with nutritional symbiosis and free-living Wigglesworthia in the milk gland organ responsible for maternal transmission to the progeny. We evaluated the reproductive fitness, longevity, digestion, and vectorial competence of flies that were devoid of Wigglesworthia. The absence of Wigglesworthia completely abolished the fertility of females but not that of males. Both the male and female Wigglesworthia-free adult progeny displayed longevity costs and were significantly compromised in their blood meal digestion ability. Finally, while the vectorial competence of the young newly hatched adults without Wigglesworthia was comparable to that of their wild-type counterparts, older flies displayed higher susceptibility to trypanosome infections, indicating a role for the mutualistic symbiosis in host immunobiology. The ability to rear adult tsetse that lack the obligate Wigglesworthia endosymbionts will now enable functional investigations into this ancient symbiosis.
Figures




References
-
- Akman, L., A. Yamashita, H. Watanabe, K. Oshima, T. Shiba, M. Hattori, and S. Aksoy. 2002. Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 32:402-407. - PubMed
-
- Aksoy, S. 1995. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int. J. Syst. Bacteriol. 45:848-851. - PubMed
-
- Aksoy, S., W. C. Gibson, and M. J. Lehane. 2003. Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Adv. Parasitol. 53:1-83. - PubMed
-
- Attardo, G. M., C. Lohs, A. Heddi, U. A. Alam, S. Yildirim, and S. Aksoy. 2008. Analysis of milk gland ultrastructure and function in Glossina morsitans: milk protein production, symbiont populations and fertility. J. Insect Physiol. [Epub ahead of print.] doi: 10.1016/j.jinsphys. 2008.06.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

