Neuraminidase-1 is required for the normal assembly of elastic fibers
- PMID: 18689602
- PMCID: PMC2575946
- DOI: 10.1152/ajplung.90346.2008
Neuraminidase-1 is required for the normal assembly of elastic fibers
Abstract
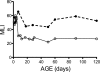
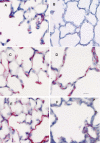
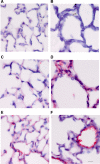
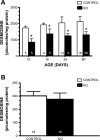
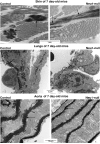
The assembly of elastic fibers in tissues that undergo repeated cycles of extension and recoil, such as the lungs and blood vessels, is dependent on the proper interaction and alignment of tropoelastin with a microfibrillar scaffold. Here, we describe in vivo histopathological effects of neuraminidase-1 (Neu1) deficiency on elastin assembly in the lungs and aorta of mice. These mice exhibited a tight-skin phenotype very similar to the Tsk mouse. Normal septation of Neu1-null mice did not occur in neonatal mice, resulting in enlarged alveoli that were maintained in adults. The abnormal development of elastic fibers was remarkable under electron microscopy and confirmed by the overlapping distribution of elastin, fibrillin-1, fibrillin-2, and fibulin-5 (Fib-5) by the light microscopy immunostainings. Fib-5 fibers appeared diffuse and unorganized around the alveolar walls and the apex of developing secondary septal crests. Fibrillin-2 deposition was also abnormal in neonatal and adult lungs. Dispersion of myofibroblasts appeared abnormal in developing lungs of Neu1-null mice, with a random distribution of myofibroblast around the alveolar walls, rather than concentrating at sites of elastin synthesis. The elastic lamellae in the aorta of the Neu1-null mice were thinner and separated by hypertrophic smooth muscle cells that were surrounded by an excess of the sialic acid-containing moieties. The concentration of elastin, as measure by desmosine levels, was significantly reduced in the aorta of Neu1-null mice. Message levels for tropoelastin and Fib-5 were normal, suggesting the elastic fiber defects in Neu1-null mice result from impaired extracellular assembly.
Figures




Similar articles
-
Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice.Mol Cell Biol. 2006 Mar;26(5):1700-9. doi: 10.1128/MCB.26.5.1700-1709.2006. Mol Cell Biol. 2006. PMID: 16478991 Free PMC article.
-
Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly.Matrix Biol. 2009 May;28(4):211-20. doi: 10.1016/j.matbio.2009.03.004. Epub 2009 Mar 24. Matrix Biol. 2009. PMID: 19321153 Free PMC article.
-
Dietary iron deficiency compromises normal development of elastic fibers in the aorta and lungs of chicks.J Nutr. 2007 Aug;137(8):1895-900. doi: 10.1093/jn/137.8.1895. J Nutr. 2007. PMID: 17634261
-
New insights into elastic fiber assembly.Birth Defects Res C Embryo Today. 2007 Dec;81(4):229-40. doi: 10.1002/bdrc.20111. Birth Defects Res C Embryo Today. 2007. PMID: 18228265 Review.
-
Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins.Adv Exp Med Biol. 2014;802:31-47. doi: 10.1007/978-94-007-7893-1_3. Adv Exp Med Biol. 2014. PMID: 24443019 Review.
Cited by
-
Regulation of intracellular signaling by extracellular glycan remodeling.ACS Chem Biol. 2010 Jan 15;5(1):35-46. doi: 10.1021/cb9002514. ACS Chem Biol. 2010. PMID: 19968325 Free PMC article. Review.
-
Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase.Glycoconj J. 2009 Dec;26(9):1197-212. doi: 10.1007/s10719-009-9239-8. Glycoconj J. 2009. PMID: 19430901
-
Thymoquinone from nutraceutical black cumin oil activates Neu4 sialidase in live macrophage, dendritic, and normal and type I sialidosis human fibroblast cells via GPCR Galphai proteins and matrix metalloproteinase-9.Glycoconj J. 2010 Apr;27(3):329-48. doi: 10.1007/s10719-010-9281-6. Epub 2010 Mar 6. Glycoconj J. 2010. PMID: 20213245
-
Characterization of novel interactions with membrane NEU1 highlights new regulatory functions for the Elastin Receptor Complex in monocyte interaction with endothelial cells.Cell Biosci. 2021 Dec 13;11(1):206. doi: 10.1186/s13578-021-00718-x. Cell Biosci. 2021. PMID: 34903296 Free PMC article.
-
The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in vitro and murine lung in vivo.Glycobiology. 2016 Aug;26(8):834-49. doi: 10.1093/glycob/cww060. Epub 2016 May 25. Glycobiology. 2016. PMID: 27226251 Free PMC article.
References
-
- Achyuthan KE, Achyuthan AM. Comparative enzymology, biochemistry and pathophysiology of human exo-alpha-sialidases (neuraminidases). Comp Biochem Physiol B Biochem Mol Biol 129: 29–64, 2001. - PubMed
-
- Bonten EJ, Arts WF, Beck M, Covanis A, Donati MA, Parini R, Zammarchi E, d'Azzo A. Novel mutations in lysosomal neuraminidase identify functional domains and determine clinical severity in sialidosis. Hum Mol Genet 9: 2715–2725, 2000. - PubMed
-
- Bonten EJ, D'Azzo A. Lysosomal neuraminidase. Catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J Biol Chem 275: 37657–37663, 2000. - PubMed
-
- Brody JS, Vaccaro C. Postnatal formation of alveoli: interstitial events and physiologic consequences. Fed Proc 38: 215–223, 1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

