Effects of Plasmodium falciparum mixed infections on in vitro antimalarial drug tests and genotyping
- PMID: 18689621
- PMCID: PMC2680026
Effects of Plasmodium falciparum mixed infections on in vitro antimalarial drug tests and genotyping
Abstract
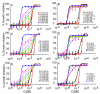
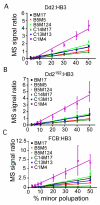
Studying drug resistance in Plasmodium falciparum requires accurate measurement of parasite response to a drug. Factors such as mixed infection of drug-resistant and -sensitive parasites can influence drug test outcome. Polymorphic DNA sequences are frequently used to detect mixed infections; infections with a single genotype or having a minor allele smaller than a subjectively selected cut-off value are often considered single infection. We studied the effects of mixed parasite populations containing various ratios of parasites resistant and sensitive to chloroquine on outcomes of drug tests and how ratios of parasite mixtures correlated with genotypes using polymerase chain reaction-based methods. Our results show that a mixture with a resistant population as low as 10% could greatly impact a drug test outcome. None of the genotyping methods could reliably detect minor DNA alleles at < or = 10%. Mixed infection presents a serious problem for drug tests, and genotyping using microsatellite or other methods may not reliably reflect true ratios of alleles.
Figures

References
-
- Yeung S, Pongtavornpinyo W, Hastings IM, Mills AJ, White NJ. Antimalarial drug resistance, artemisinin-based combination therapy, and the contribution of modeling to elucidating policy choices. Am J Trop Med Hyg. 2004;71:179–186. - PubMed
-
- Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. Drug sensitivity of Plasmodium falciparum. An in-vitro microtechnique. Lancet. 1978;1:22–23. - PubMed
-
- Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
