Membrane curvature induced by Arf1-GTP is essential for vesicle formation
- PMID: 18689681
- PMCID: PMC2575275
- DOI: 10.1073/pnas.0805182105
Membrane curvature induced by Arf1-GTP is essential for vesicle formation
Abstract
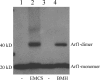
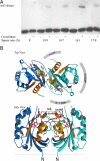
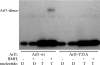
The GTPase Arf1 is considered as a molecular switch that regulates binding and release of coat proteins that polymerize on membranes to form transport vesicles. Here, we show that Arf1-GTP induces positive membrane curvature and find that the small GTPase can dimerize dependent on GTP. Investigating a possible link between Arf dimerization and curvature formation, we isolated an Arf1 mutant that cannot dimerize. Although it was capable of exerting the classical role of Arf1 as a coat receptor, it could not mediate the formation of COPI vesicles from Golgi-membranes and was lethal when expressed in yeast. Strikingly, this mutant was not able to deform membranes, suggesting that GTP-induced dimerization of Arf1 is a critical step inducing membrane curvature during the formation of coated vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. - PubMed
-
- Serafini T, et al. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: A novel role for a GTP-binding protein. Cell. 1991;67:239–253. - PubMed
-
- D'Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. - PubMed
-
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

