Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus
- PMID: 18689690
- PMCID: PMC2504482
- DOI: 10.1073/pnas.0803711105
Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus
Abstract
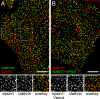
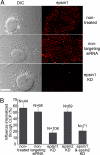
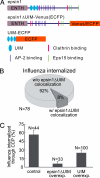
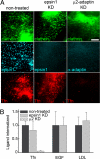
During clathrin-mediated endocytosis, adaptor proteins recognize specific internalization signals on cargo receptors, either recruiting cargos into clathrin-coated pits (CCPs) or initiating clathrin-coat assembly around the cargo molecules. Here, we identify epsin 1, a clathrin-, ubiquitin-, and phospholipid-interacting protein, as a cargo-specific adaptor for influenza virus entry through the clathrin-mediated pathway. Using live-cell imaging to monitor the entry of individual virus particles, we observed recruitment of epsin 1 to the binding sites of influenza viruses in synchrony with the assembly of CCPs. Epsin 1 knockdown by siRNA significantly inhibited the clathrin-mediated endocytosis of the influenza virus and caused the majority of the virus particles to enter through a clathrin-independent pathway. The same treatment did not affect the entry of several classical ligands for clathrin-mediated endocytosis, including transferrin, LDL, and EGF. Overexpression of the dominant-negative epsin 1 mutant lacking the ubiquitin-interaction motifs nearly completely blocked the clathrin-mediated entry of the influenza virus without affecting transferrin uptake. These results suggest that epsin 1 functions as a cargo-specific adaptor for the clathrin-mediated entry of the influenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: Shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. - PubMed
-
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

