Human neural crest cells display molecular and phenotypic hallmarks of stem cells
- PMID: 18689800
- PMCID: PMC2566525
- DOI: 10.1093/hmg/ddn235
Human neural crest cells display molecular and phenotypic hallmarks of stem cells
Abstract
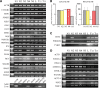
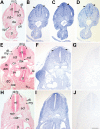
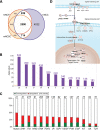
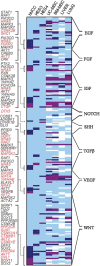
The fields of both developmental and stem cell biology explore how functionally distinct cell types arise from a self-renewing founder population. Multipotent, proliferative human neural crest cells (hNCC) develop toward the end of the first month of pregnancy. It is assumed that most differentiate after migrating throughout the organism, although in animal models neural crest stem cells reportedly persist in postnatal tissues. Molecular pathways leading over time from an invasive mesenchyme to differentiated progeny such as the dorsal root ganglion, the maxillary bone or the adrenal medulla are altered in many congenital diseases. To identify additional components of such pathways, we derived and maintained self-renewing hNCC lines from pharyngulas. We show that, unlike their animal counterparts, hNCC are able to self-renew ex vivo under feeder-free conditions. While cross species comparisons showed extensive overlap between human, mouse and avian NCC transcriptomes, some molecular cascades are only active in the human cells, correlating with phenotypic differences. Furthermore, we found that the global hNCC molecular profile is highly similar to that of pluripotent embryonic stem cells when compared with other stem cell populations or hNCC derivatives. The pluripotency markers NANOG, POU5F1 and SOX2 are also expressed by hNCC, and a small subset of transcripts can unambiguously identify hNCC among other cell types. The hNCC molecular profile is thus both unique and globally characteristic of uncommitted stem cells.
Figures




References
-
- Abzhanov A., Tzahor E., Lassar A.B., Tabin C.J. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–4579. - PubMed
-
- Couly G., Creuzet S., Bennaceur S., Vincent C., Le Douarin N.M. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. - PubMed
-
- McGonnell I.M., Graham A. Trunk neural crest has skeletogenic potential. Curr. Biol. 2002;12:767–771. - PubMed
-
- Le Douarin N., Kalcheim C. The Neural Crest. Cambridge, UK: Cambridge University Press; 1999.
-
- Le Lievre C.S., Le Douarin N.M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 1975;34:125–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

